Abstract
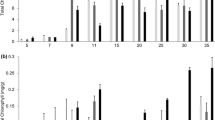
Infective (nodulating) Rhizobium leguminosarum biovar viciae (R.l. viciae) bacteria release Nod factors which stimulate the release of nodulation gene-inducing flavanones and chalcones from roots of the host plant Vicia sativa subsp. nigra (K. Recourt et al., Plant Mol Biol 16: 841–852; H.P. Spaink et al., Nature 354: 125–130). The hypothesis that this release results from increased synthesis of flavonoids was tested by studying the effect of inoculation of V. sativa with infective and uninfective R.l. viciae bacteria on (i) activity of L-phenylalanine ammonia-lyase, (ii) level of chalcone synthase mRNA, and (iii) activity of (eriodictyol) methyltransferase in roots. Consistent with the hypothesis, each of these parameters was found to increase 1.5 to 2-fold upon inoculation with infective R.l. viciae bacteria relative to the situation for uninoculated roots and for roots inoculated with uninfective rhizobia.
Similar content being viewed by others
References
Bolwell GP, Bell JN, Cramer CL, Schuch W, Lamb CJ, Dixon RA: L-Phenylalanine ammonia-lyase from Phaseolus vulgaris: Characterization and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem 149: 411–419 (1985).
Dixon RA, Lamb CJ: Molecular communication in interactions between plants and microbial pathogens. Annu Rev Plant Physiol Plant Mol Biol 41: 339–367 (1990).
Djordjevic MA, Redmond JW, Batley M, Rofle BG: Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J 6: 1173–1179 (1987).
Estabrook EM, Sengupta-Gopalan C: Differential expression of phenylalanine ammonia-lyase and chalcone synthase during soybean nodule development. Plant Cell 3: 299–308 (1991).
Firmin JL, Wilson KE, Rossen L, Johnston AWB: Flavonoid activation of nodulation genes in Rhizobium reversed by other compounds present in plants. Nature 324: 90–92 (1986).
Hanson KR, Havir EA: Phenylalanine Ammonia-Lyase. In: Conn EE (ed) The Biochemistry of Plants, vol 7: Secondary Plant Products, pp 577–625. Academic Press, London/ New York (1981).
Heller W, Forkmann G: Biosynthesis. In: Harborne JB (ed). The flavonoids, advances in research since 1980. pp 399–427. Chapman and Hall London/ New York (1988).
Hooykaas PJJ, van Brussel AAN, den Dulk-Ras H, van Slogteren GMS, Schilperoort RA: Sym plasmid of Rhizobium trifolii expressed in different rhizobial species and Agrobacterium tumefaciens. Nature 291: 351–353 (1981).
Horvath B, Bachem CW, Schell J, Kondorosi A: Hostspecific regulation of nodulation genes in Rhizobium is mediated by a plant signal interacting with the nodD gene product. EMBO J 6: 841–848 (1987).
Hungria M, Joseph CM, Phillips DA: Rhizobium nod gene inducers exuded naturally from roots of common bean (Phaseolus vulgaris L.). Plant Physiol 97: 759–764 (1991).
Jacobs J, Rubery PH: Naturally occurring auxin transport regulators. Science 241: 346–349 (1988).
Koes RE, Spelt CE, Reif HJ, van den Elzen PJM, Veltkamp E, Mol JNM: Floral tissue of Petunia hybrida (V30) expresses only one member of the chalcone synthase multigene family. Nucl Acids Res 14: 5229–5239 (1987).
Kosslak RM, Bookland R, Barkei J, Paaren HE, Appelbaum ER: Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci USA 84: 7428–7432 (1987).
Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Prome JC, Denarie J: Symbiotic host specifity of Rhizobium meliloti is determined by a sulphated and acetylated glucosamine oligosaccharide signal. Nature 344: 781–784 (1990).
Marbry TJ, Markham KR, Thomas MB: The Systematic Identification of Flavonoids. Springer-Verlag, Berlin/Heidelberg/New York (1970).
Maxwell CA, Hartwig UA, Joseph CM, Phillips DA: A chalcone and two related flavonoids released from alfalfa roots induce nod genes of Rhizobium meliloti. Plant Physiol 91: 842–847 (1989).
Maxwell CA, Phillips DA: Concurrent synthesis and release of nod-gene-inducing flavonoids from alfalfa roots. Plant Physiol 93: 1552–1558 (1990).
Peters NK, Long SR: Alfalfa root exudates and compounds which promote or inhibit induction of Rhizobium meliloti nodulation genes. Plant Physiol 88: 396–400 (1988).
Poulton JE, Hahlbrock K, Griesebach H: O-Methylation of flavonoid substrates by a partially purified enzyme from soybean cell suspension cultures. Arch Biochem Biophys 180: 543–549 (1977).
Recourt K, Schripsema J, Kijne JW, van Brussel AAN, Lugtenberg BJJ: Inoculation of Vicia sativa subsp. nigra roots with Rhizobium leguminosarum biovar viciae results in release of nod gene activating flavanones and chalcones. Plant Mol Biol 16: 841–852 (1991).
Recourt K, Verkerke M, Schripsema J, van Brussel AAN, Lugtenberg BJJ, Kijne JW: Major flavonoids in roots of uninoculated and inoculated roots of Vicia sativa subsp. nigra are four conjugates of the nodulation gene-inhibitor kaempferol. Plant Mol Biol, (in press).
Recourt K, van Brussel AAN, Driessen AJM, Lugtenberg BJJ: Accumulation of a nod gene inducer, the flavonoid naringenin, in the cytoplasmic membrane of Rhizobium leguminosarum biovar viciae is caused by the pH-dependent hydrophobicity of naringenin. J Bact 171: 4370–4377 (1989).
Redmond JW, Batley M, Djordevic MA, Innes RW, Kuempel PL, Rolfe BG: Flavones induce expression of nodulation genes in Rhizobium. Nature 323: 632–635 (1986).
Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJJ: Regulation of the promoters in the nodulation region of the symbiosis plasmid pRL1J1 of Rhizobium leguminosarum. In: Verma DPS, Brisson N (eds) Molecular Genetics of Plant-Microbe Interactions, pp. 244–246. Martinus Nijhoff, Dordrecht (1987).
Spaink HP, Sheeley DM, van Brussel AAN, Glushka J, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg BJJ: A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 354: 124–130 (1991).
van Brussel AAN, Zaat SAJ, Canter Cremerg HCJ, Wijffelman CA, Pees E, Tak T, Lugtenberg BJJ: Role of plant root exudate and sym plasmid-localized nodulation genes in the synthesis by Rhizobium leguminosarum of Tsr factor which causes thick and short roots on common vetch. J Bact 165: 517–522 (1986).
van Brussel AAN, Recourt K, Pees E, Spaink HP, Tak T, Wijffelman CA, Kijne JW, Lutgenberg BJJ: A biovar-specific signal of Rhizobium leguminosarum biovar viciae induces increased nodulation gene-inducing activity in root exudate of Vicia sativa subsp. nigra. J Bact 172: 5394–5401 (1990).
van Iren F, van der Knaap M, van den Heuvel, Kijne JW: Phytoalexins and nodulation in Pisum sativum. In: C Veeger, WE Newton (eds) Advances in Nitrogen Fixation Research, p. 433 Nijhoff/Junk, The Hague/Pudoc, Wageningen (1984).
Vansumere K, Vandecasteele K, Hutsebaut W, Evaert E, de Cooman L, Meulemans W: RP-HPLC Analysis of flavonoids and the biochemical identification of hop cultivars. In: Carl H (ed) Proceedings of the EBC Symposium on Hops, pp. 146–175. Brauwelt Verlag, Nuernberg (1988).
van Tunen AJ, Koes RE, Spelt CE, van der Krol AR, Stuitje AR, Mol JNM: Cloning of the two chalcone flavanone isomerase genes from Petunia hybrida; coordinated, light-regulated and differential expression of flavonoid genes. EMBO J 7: 1257–1263 (1988).
Werner D, Mellor RB, Hahn MG, Grisebach H: Soybean root response to symbiotic infection: Glyceollin I accumulation in an ineffective type of soybean nodules with an early loss of the peribacteroid membrane. Z Naturforsch 40c: 179–181 (1985).
Wijffelman C, Zaat B, Spaink H, Mulders I, van Brussel A, Okker R, de Maagd R, Lugtenberg B: Induction of Rhizobium nod genes by flavonoids: differential adaptation of promoter, nodD gene and inducers for various cross-inoculation groups. In: Lugtenberg B (ed) Recognition in Microbe-Plant Symbiotic and Pathogenic Interactions, ATO ASI Series, Vol H 4, pp. 123–135. Springer-Verlag, Berlin/Heidelberg (1986).
Zaat SAJ, Wijffelman CA, Spaink HP, van Brussel AAN, Oker RJH, Lugtenberg BJJ: Induction of the nodA promoter of Rhizobium leguminosarum Sym plasmid pRL1J1 by plant flavanones and flavones. J Bact 169: 198–204 (1987).
Zaat SAJ, Schripsema J, Wijffelman CA, van Brussel AAN, Lugtenberg BJJ: Analysis of the major inducers of the Rhizobium nodA promoter from Vicia sativa root exudate and their activity with different nodD genes. Plant Mol Biol 13: 175–188 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Recourt, K., van Tunen, A.J., Mur, L.A. et al. Activation of flavonoid biosynthesis in roots of Vicia sativa subsp. nigra plants by inoculation with Rhizobium leguminosarum biovar viciae . Plant Mol Biol 19, 411–420 (1992). https://doi.org/10.1007/BF00023389
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00023389