Abstract
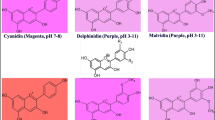
Cell cultures of grapes, Vitis vinifera L. cv Gamay Fréaux were grown under different conditions of external osmotic potential induced by an increase of sucrose concentration or by the addition of mannitol to the culture medium. Addition of 82 mM mannitol or increasing sucrose concentration to 132 mM had similar effects on repressing growth. Cyanidin 3-glucoside, peonidin 3-glucoside and peonidin 3-p-coumaroylglucoside are three main anthocyanins of Vitis cells. Increasing osmotic potential from −0.43 MPa to −0.8 MPa in the medium resulted in a significant intracellular accumulation of anthocyanin especially peonidin 3-glucoside in the pigmented cells. High osmotic potential appears to stimulate the methylation of anthocyanins. Osmotic potential is an important culture factor and may be useful in the controlling of anthocyanin production and composition.
Similar content being viewed by others
References
Amino SI & Tazawa M (1988) Uptake and utilization of sugars in cultured rice cells. Plant Cell Physiol. 29:483–488
Bakker J & Timberlake CF (1985) The distribution of anthocyanins in grape skin extracts of Port Wine cultivars as determined by High Performance Liquid Chromatography. J. Sci. Food Agric. 36: 1315–1324
Cormier F, Crevier H & Do CB (1990) Effects of sucrose concentration on the accumulation of anthocyanins in grape (Vitis vinifera L.) cell suspension. Can. J. Bot. 68: 1822–1826
Dougall DK & Frazier GC (1989) Nutrient utilization during biomass and anthocyanin accumulation in suspension cultures of wild carrot cells. Plant Cell Tiss. Org. Cult. 18: 95–104
Francis IF (1982) Analysis of anthocyanins. In: Markakis P (Ed) Anthocyanins as Food Colors (pp 181–208). Academic Press, London
Francis JF (1989) Food colorant: Anthocyanins. In: Critical Review in Food Science and Nutrition. CRC Press 28: 273–314
Fuleki T & Somerville RG (1984) Separation of anthocyanins of Concord grapes by high performance liquid chromatography. Bull. Liaison Groupe Polyphénols 12: 506–512
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res. 50: 151–158
Handa AK, Bressan RA, Handa H & Hasegawa PM (1982) Characteristics of cultured tomato cells after prolonged exposure to medium containing polyethylene glycol. Plant Physiol. 69: 514–521
Harbone JB & Grayer RJ (1988) The anthocyanins. In: Harbone JB (Ed) The Flavonoids: Advances in Research Since 1980 (pp 1–20). Chapman and Hall Ltd
Heller W & Forkmann G (1988) Biosynthesis. In: Harbone JB (Ed) The Flavonoids: Advances In Research Since 1980 (pp 399–425). Chapman and Hall Ltd
Hirose M, Yamakawa T, Kodama T, Komamine A (1990) Accumulation of betacyanin in Phytolacca americana and of anthocyanin in Vitis sp. cells in relation to cell division in suspension cultures. Plant Cell Physiol. 31: 267–271
Ibrahim RK (1987) Regulation of synthesis of phenolics. In: Constabel F & Vasil IK (Eds) Cell Culture and Somatic Cell Genetics of Plant. Cell Culture Phytochem. 4: 77–89
Ilker R (1987) In vitro pigment production: an alternative to color synthesis. Food Tech. 41: 70–72
Karppa J, Kallio H, Peltonen I & Linko R (1984) Anthocyanins of crowberry, Empetrum nigrum coll. J. Food Sci. 49: 634–636
La Presse (1990) Colorant cancérigène. No 106 Cahier C. 2P
Lofty S, Fleuriet A, Ramos T & Macheix JJ (1989) Biosynthesis of phenolic compounds in Vitis vinifera cell suspension cultures: Study on Hydroxycinnamoyl CoA ligase. Plant Cell Rep. 8: 93–96
Matsumoto T, Nishida K, Noguchi M & Tamaki E (1973) Some factors affecting the anthocyanin formation by Populus cells in suspension culture. Agric. Biol. Chem. 37: 561–567
Nozue M, Kazwai J & Yoshitama K (1987) Selection of a high anthocyanin-producing cell line of sweet potato cell cultures and identification of pigments. J. Plant Physiol. 129: 81–88
Ozeki Y & Komamine A (1985) Effects of inoculum density, zeatin and sucrose on anthocyanin accumulation in a carrot suspension culture. Plant Cell Tiss. Org. Cult. 5: 45–53
Salisbury FB & Ross CW (1985) Osmosis. In: Carey JC (Ed) Plant Physiology (pp 33–53). Wadsworth, Inc.
SAS (1987) SAS/STAT Guide For Personal Computer, version 6. (Ed) SAS Institute Inc. NC, USA
Tamura H, Kumaoka Y & Sugisawa H (1989) Identification and quantitative variation of anthocyanins produced by cultured callus tissue of Vitis sp. Agric. Biol. Chem. 53: 1969–1970
Van Calsteren MR, Cormier F, Do CB & Laing R (1990) 1H and 13C assignments of the major anthocyanins from Vitis vinifera cell suspension culture. Can. J. Chem. (submitted)
William M & Hrazdina G (1978) High-Pressure Liquid Chromatographic separation of 3-glucoside, 3,5-diglucoside, 3-(6-o-p-coumaryl) glucoside and 3-(6-o-p-coumarylglucoside)-5-glucoside of anthocyanins. J. Chromatogr. 155: 389–398
Wulf LW & Nagel CW (1978) High-Pressure Liquid Chromatographic separation of anthocyanins of Vitis vinifera. Am. J. Enol. Vit. 29: 42–49
Yamakawa T, Sato S, Ishida K, Kodama T & Minoda Y (1983) Production of anthocyanins by Vitis cells in suspension culture. Agric. Biol. Chem. 47: 2185–2191
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Do, C.B., Cormier, F. Accumulation of peonidin 3-glucoside enhanced by osmotic stress in grape (Vitis vinifera L.) cell suspension. Plant Cell Tiss Organ Cult 24, 49–54 (1991). https://doi.org/10.1007/BF00044265
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00044265