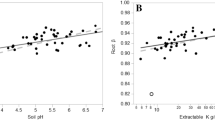
Abstract
Understanding and predicting ecosystem functioning (e.g., carbon and water fluxes) and the role of soils in carbon storage requires an accurate assessment of plant rooting distributions. Here, in a comprehensive literature synthesis, we analyze rooting patterns for terrestrial biomes and compare distributions for various plant functional groups. We compiled a database of 250 root studies, subdividing suitable results into 11 biomes, and fitted the depth coefficient β to the data for each biome (Gale and Grigal 1987). β is a simple numerical index of rooting distribution based on the asymptotic equation Y=1-βd, where d = depth and Y = the proportion of roots from the surface to depth d. High values of β correspond to a greater proportion of roots with depth. Tundra, boreal forest, and temperate grasslands showed the shallowest rooting profiles (β=0.913, 0.943, and 0.943, respectively), with 80–90% of roots in the top 30 cm of soil; deserts and temperate coniferous forests showed the deepest profiles (β=0.975 and 0.976, respectively) and had only 50% of their roots in the upper 30 cm. Standing root biomass varied by over an order of magnitude across biomes, from approximately 0.2 to 5 kg m-2. Tropical evergreen forests had the highest root biomass (5 kg m-2), but other forest biomes and sclerophyllous shrublands were of similar magnitude. Root biomass for croplands, deserts, tundra and grasslands was below 1.5 kg m-2. Root/shoot (R/S) ratios were highest for tundra, grasslands, and cold deserts (ranging from 4 to 7); forest ecosystems and croplands had the lowest R/S ratios (approximately 0.1 to 0.5). Comparing data across biomes for plant functional groups, grasses had 44% of their roots in the top 10 cm of soil. (β=0.952), while shrubs had only 21% in the same depth increment (β=0.978). The rooting distribution of all temperate and tropical trees was β=0.970 with 26% of roots in the top 10 cm and 60% in the top 30 cm. Overall, the globally averaged root distribution for all ecosystems was β=0.966 (r 2=0.89) with approximately 30%, 50%, and 75% of roots in the top 10 cm, 20 cm, and 40 cm, respectively. We discuss the merits and possible shortcomings of our analysis in the context of root biomass and root functioning.
Similar content being viewed by others
References
Ågren GI, Axelsson B, Flower-Ellis JGK, Linder S, Persson H, Staaf H, Troeng E 1980. Annual carbon budget for a young Scots pine. Ecol Bull 32:307–313
Allen M (1991) The ecology of mycorrhizae. Cambridge University Press, Cambridge
Archer S (1995) Tree-grass dynamics in a subtropical savanna: reconstructing the past, predicting the future. Ecoscience 2:83–99
Böhm W (1979) Methods of studying root systems. Springer Berlin Heidelberg New York
Bonan GB (1992) Soil temperature as an ecological factor in boreal forests. In: a systems analysis of the global boreal forest. Shugart HH, Leemans R, Bonan GB (eds) Cambridge University Press, Cambridge, pp 126–143
Burke IC, Kittel TGF, Lauenroth WK, Snook P, Yonker CM, Parton WJ (1991) Regional analysis of the Central Great Plains. BioScience 41:685–692
Buxton PA (1925) The temperature of the surface of deserts. J Ecol 12:127–134
Caldwell MM, Richards JH (1986) Competing root systems: morphology and models of absorption. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 251–273
Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) Maximum rooting depth for vegetation types at the global scale. Oecologia, in press
Cannon WA (1911) The root habits of desert plants (Publication 131). Carnegie Institution, Washington
Chapin FS III, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J (1992) Arctic ecosystems in a changing climate. Academic Press, San Diego
Coleman DC (1976) A review of root production processes and their influence on soil biota in terrestrial ecosystems. In: Macfadyen JMA (ed) The role of terrestrial and aquatic organisms in decomposition processes. Blackwell, Oxford
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Dickinson RE, Henderson-Sellers A (1988) Modelling tropical deforestation: study of GCM land-surface parameterizations. Q J Meteorol Soc 114:439–462
Dobrowolski JP, Caldwell MM, Richards JH (1990) Basin hydrology and plant root systems. In: Osmond CB, Pitelka LF, Hidy GM (eds) Plant biology of the Basin and Range. Springer Berlin Heidelberg New York, pp 243–292
Drew MC (1990) Sensing soil oxygen. Plant Cell Environ 13:681–693
Evenari M, Shanan L, Tadmore N (1971) The Negev: challenge of a desert. Harvard University Press, Cambridge
Farrish KW (1991) Spatial and temporal fine-root distribution in three Louisiana forest soils. Soil Sci Soc Am J 55:1752–1757
Field CB, Jackson RB, Mooney HA (1995) Stomatal responses to increased CO2: implications from the plant to the global scale. Plant Cell Environ 18:1214–1225
Fisher MJ, Rao IM, Ayarza MA, Lascano CE, Sanz JI, Thomas RJ, Vera RR (1994) Carbon storage by introduced deep-rooted grasses in the South American savannas. Nature 371:236–238
Fitter AH (1982) Morphometric analysis of root systems: application of the technique and influence of soil fertility on root system development in two herbaceous species. Plant Cell Environ 5:313–322
Fox RL, Lipps RC (1964) A comparison of stable strontium and 32P and tracers for estimating alfalfa root activity. Plant Soil 20:337–350
Freckman DW (1995) Life in the soil: soil biodiversity and its importance to ecosystem processes. The Natural History Museum, London
Freckman DW, Virginia RA (1989) Plant-feeding nematodes in deep-rooting desert ecosystems. Ecology 70:1665–1678
Gale MR, Grigal DF (1987) Vertical root distributions of northern tree species in relation to successional status. Can J For Res 17:829–834
Gile LH, Peterson FF, Grossman RB (1966) Morphological and genetic sequences of carbonate accumulation in desert soils. Soil Sci 101:347–360
Golluscio RA, Sala OE (1993) Plant functional types and ecological strategies in Patagonian forbs. J Veg Sci 4:839–846
Grier CC, Vogt KA, Keyes MR, Edmonds RL (1981) Biomass distribution and above-and below-ground production in young and mature Abies amabilis zone ecosystems of the Washington Cascades. Can J For Res 11:155–167
Hales S (1727) Vegetable staticks, current edition (1961). London Scientific Book Guild, London
Hall NS, Chandler WF, Bavel CHM van, Reid PH, Anderson JH (1953) A tracer technique to measure growth and activity of plant root systems. N C Agric Exp Sta Tech Bull 101:1–40
Hawksworth DL, Ritchie JM (1993) Biodiversity and biosystematic priorities: microorganisms and invertebrates. CAB International, Wallingford
Heitschmidt RK, Ansley RJ, Dowhower SL, Jacoby PW, Price DL (1988) Some observations from the excavation of honey mesquite root systems. J Range Manage 41:227–230
Hendrick RL, Pregitzer KS (1993) Patterns of fine root mortality in two sugar maple forests. Nature 361:59–61
Higgins KB, Lamb AJ, Wilgen BW van (1987) Root systems of selected plant species in mesic fynbos in the Jonkershoek Valley, south-western Cape Province. S Afr J Bot 53:249–257
Hilbert DW, Canadell J (1966) Biomass partitioning and resource allocation of plants from Mediterranean-type ecosystems: posible responses to elevated atmospheric CO2. In: Moreno JM, Oechel WC (eds) Global change and mediterranean-type ecosystems, Ecological studies 117, Springer Berlin Heidelberg New York, pp 76–101
Holmes JW, JS Colville (1970) Forest hydrology in a karstic region of southern Australia. J Hydrol 10:59–74
Jackson RB, Caldwell MM 1993. Geostatistical patterns of soil heterogeneity around individual perennial plants. J Ecol 81:683–692
Jackson RB, Manwaring JH, Caldwell MM (1990) Rapid physiological adjustment of roots to localized soil enrichment. Nature 344:58–60
Kane DL, Hinzman LD, Woo M, Everett KR (1992) Arctic hydrology and climate change. In: III Chapin FS, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J (eds) Arctic ecosystems in a changing climate. Academic Press, San Diego, pp 35–51
Klinge H (1973) Root mass estimation in lowland tropical rain forests of central Amazonia, Brazil. I. Fine root masses of a pale yellow latosol and a giant humus podzol. Trop Ecol 14:29–38
Klinge H, Herrera R (1978) Root biomass studies in Amazon caatinga forest in southern Venezuela. I. Standing crop of composite root mass in selected stands. Trop Ecol 19:93–110
Kochenderfer JN (1973) Root distribution under some forest types native to West Virginia. Ecology 54:445–448
Kummerow J (1981) Structure of roots and root systems. In: Castri F di, Goodall DW, Specht RL (eds) Mediterranean-Type Shrublands, Elsevier, New York, pp 269–288
Kummerow J, Mangan R (1981) Root systems in Quercus dumosa Nutt. dominated chaparral in southern California. Acta Oecol 2:177–188
Kummerow J, Krause D, Jow W (1977) Root systems of chaparral shrubs. Oecologia 29:163–177
Lean J, Warrilow DA (1989) Simulation of the regional climatic impact of Amazon deforestation. Nature 342:411–413
Le Roux X, Bariac T, Mariotti A (1995) Spatial partitioning of the soil water resource between grass and shrub components in a West African humid savanna. Oecologia 104:147–155
McKane RB, Grigal DF, Russelle MP (1990) Spatial and temporal differences in 15N uptake and the organization of an old-field plant community. Ecology 71:1126–1132
Melillo JM, McGuire AD, Kicklighter DW, Moore B III, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Nepstad DC, Carvalho CR de, Davidson EA, Jipp PH, Lefebvre PA, Negreiros GH, Silva ED da, Stone TA, Trumbore SE, Vieira S (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:66–669
Newman EI (1974) Root and soil water relations. In: Carson EW (ed) The plant root and its environment. University Press of Virginia, Charlottesville, pp 363–440
Neilson RP (1995) A model for predicting continental-scale vegetation distribution and water balance. Ecol Appl 5:362–385
Nobel PS (1988) Environmental biology of agaves and cacti. Cambridge University Press, New York
Nobel PS (1989) Temperature, water availability, and nutrient levels at various soil depths — consequences for shallow-rooted desert succulents, including nurse plant effects. Am J Bot 76:1486–1492
Oechel WC, Cowles S, Grulke N, Hastings SJ, Lawrence B, Prudhomme T, Riechers G, Strain B, Tissue D, Vourlitis G (1994) Transient nature of CO2 fertilization in Arctic tundra. Nature 371:500–503
O'Toole JC, Bland WL (1987) Genotypic variation in crop plant root systems. Adv Agron 41:91–145
Parton WJ, Stewart JWB, Cole CV (1988) Dynamics of C, N, P, and S in grassland soils: a model. Biogeochemistry 5:109–131
Parton WJ, McKeown B, Kirchner V, Ojima D (1992) Century Users Manual. Natural Resource Ecology Laboratory, Colorado State University, Fort Collins
Paruelo JM, Sala OE (1995) Water losses in the Patagonian steppe: a modelling approach. Ecology 76:510–520
Phillips WS (1963) Depth of roots in soil. Ecology 44:424
Polley HW, Mayeux HS, Johnson JB, Tischler CR (1996) Implications of rising atmospheric CO2 concentration for soil water availability and shrub/grass ratios on grasslands and savannas. J Range Manage, in press
Potter CS, Randerson JT, Field CB, Matson PA, Vitousek PM, Mooney HA, Klooster SA (1993) Terrestrial ecosystem production: a process model based on global satellite and surface data. Global Biogeochem Cycles 7:811–841
Pregitzer KS, Hendrick RL, Fogel R (1993) The demography of fine roots in response to patches of water and nitrogen. New Phytol 125:575–580
Prentice IC, Cramer W, Harrison SP, Leemans R, Monserud RA, Solomon AM (1992) A global biome model based on plant physiology and dominance, soil properties and climate. J Biogeogr 19:117–134
Raich JW, Nadelhoffer KJ (1989) Belowground carbon allocation in forest ecosystems: global trends. Ecology 70:1346–1354
Raich JW, Rastetter EB, Melillo JM, Kicklighter DW, Steudler PA, Peterson BJ, Grace AL, Moore B III, Vörösmarty CJ (1991) Potential net primary productivity in South America: application of a global model. Ecol Appl 1:399–429
Reich PB, Teskey RO, Johnson PS, Hinckley TM (1980) Periodic root and shoot growth in oak. For Sci 26:590–598
Reichle DE, Dinger BE, Edwards NT, Harris WF, Sollins P (1973) Carbon flow and storage in a forest ecosystem in: Woodwell GM, Pecan EV (eds) Carbon and the biosphere. US Atomic Energy Commission, Brookhaven Symposium in Biology, AEC Conf-720510, pp 345–365
Richards JH (1986) Root form and depth distribution in several biomes. In: Carlisle D, Berry WL, Kaplan IR, Watterson JR (eds) Mineral exploration: biological systems and organic matter. Prentice-Hall, Englewood Cliffs, pp 82–97
Richter DD, Markewitz D (1995) How deep is soil? BioScience 45:600–609
Risser PG, Birney EC, Blocker HD, May SW, Parton WJ, Wiens JA (eds) (1981) The true prairie ecosystem. (US/IBP synthesis series, vol 16). Hutchinson Ross, Stroudsburg
Rodin LE, Basilevich NI (1966) The biological productivity of the main vegetation types in northern hemisphere of the Old World. For Abstr 27:369–372
Rodin LE, Basilevich NI (1967) Production and mineral cycling in terrestrial vegetation. Oliver and Boyd, Edinburgh
Rundel PW, Nobel PS (1991) Structure and function in desert root systems. In: Atkinson D, Plant root growth: an ecological perspective. Blackwell, Oxford, pp 349–378
Running SW, Hunt ER Jr (1993) Generalization of a forest ecosystem process model for other biomes, BIOME-BGC, and an application for global-scale models. In: Ehleringer JR, Field CB (eds) Scaling physiological processes: leaf to globe. Academic Press, San Diego, pp 141–158
Sachs J (1873) Uber das Wachstum der Haupt-und Nebenwurzeln. Arb Bot Inst Wurzburg 3:395–477
Santantonio D, Hermann RK, Overton WS (1977) Root biomass studies in forest ecosystems. Pedobiologia 17:1–31
Schlesinger WH (1991) Biogeochemistry: an analysis of global change. Academic Press, San Diego
Schubart A (1857) Ueber die Wurzelbildung der Cerealien, beobachtet bei Ausspulungen derselben in ihren verschiedenen Lebensperioden. Hoffmann, Leipzig
Schulze E-D, Bauer G, Buchmann N, Canadell J, Ehleringer JR, Jackson RB, Jobbagy E, Loreti J, Mooney HA, Oesterheld M, Sala OE (1996) Water availability, rooting depth, and vegetation zones along an aridity gradient in Patagonia. Oecologia, in press
Shaver GR, Billings WD (1975) Root production and root turnover in a wet tundra ecosystem, Barrow, Alaska. Ecology 56: 401–409
Solomon AM (1992) The nature and distribution of past, present and future boreal forests: lessons for a research and modeling agenda. In: Shugart HH, Leemans R, Bonan GB (eds) A systems analysis of the global boreal forest. Cambridge University Press, New York, pp 291–301
Stone EL, Kalisz PJ (1991) On the maximum extent of tree roots. For Ecol Manage 46:59–102
Taylor HM (1987) Minirhizotron observation tubes: methods and applications for measuring rhizosphere dynamics (American Society of Agronomy special publication 50). American Society of Agronomy, Madison
Van Rees KCJ, Comerford NB (1986) Vertical root distribution and strontium uptake of a slash pine stand on a Florida spodosol. Soil Sci Soc Am J 50:1042–1046
Viereck LA, Van Cleve K, Dyrness CT (1986) Forest ecosystem distribution in the taiga environment. In: Van Cleve K, Chapin FS III, Flanagan PW, Viereck LA, Dyrness CT (eds) Forest ecosystems in the Alaskan taiga. Springer Berlin Heidelberg New York, pp 22–43
Vincent JM (1974) Root-nodule symbioses with Rhizobium. In: Quispel A (ed) The biology of nitrogen fixation. North-Holland Publishing, Amsterdam, pp 266–307
Vitousek PM, Matson PA (1984) Mechanisms of nitrogen retention in forest ecosystems: a field experiment. Sci 225:51–52
Vogt KA, Bloomfield J (1991) Tree root turnover and senescence. In: Waisel AEY, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York, pp 281–306
Vogt KA, Vogt DJ, Boon P, O'Hara J, Asbjornsen H (1996) Factors controlling the contribution of roots to ecosystem carbon cycles in boreal, temperate and tropical forests. Plant Soil, in press
Watts SE (1993) Rooting patterns of co-occurring woody plants on contrasting soils in a subtropical savanna. MS dissertation, Texas A&M University, College Station
Weaver JE (1926) Root development of field crops. McGraw-Hill, New York
Wilson MF, Henderson-Sellers A (1985) A global archive of land cover and soils data for use in general circulation models. J Climatol 5:119–143
Wullschleger SD, Lynch JP, Berntson GM (1994) Modeling the belowground response of plants and soil biota to edaphic and climatic change: what can we expect to gain. Plant Soil 165: 149–160
Zohary M (1961) On the hydro-ecological relations of the near east desert vegetation. UNESCO Arid Zone Res 16:198–212
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jackson, R.B., Canadell, J., Ehleringer, J.R. et al. A global analysis of root distributions for terrestrial biomes. Oecologia 108, 389–411 (1996). https://doi.org/10.1007/BF00333714
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00333714