Abstract
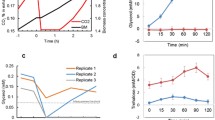
Micro-organisms have developed systems to adapt to sudden changes in the environment. Here we describe the response of the yeastSaccharomyces cerevisiae to osmotic stress. A drop in the water activity (aw) of the medium following the addition of NaCl led to an immediate shrinkage of the cells. During the 2 h following the osmotic shock the cells partially restored their cell volume. This process depended on active protein synthesis. During the recovery period the cells accumulated glycerol intracellularly as a compatible solute and very little glycerol was leaking out of the cell. We have investigated in more detail the enzymes of glycerol metabolism and found that only the cytoplasmic glycerol-3-phosphate dehydrogenase was strongly induced. The level of induction was dependent on the yeast strain used and the degree of osmotic stress. The synthesis of cytoplasmic glycerol-3-phosphate dehydrogenase is also regulated by glucose repression. Using mutants defective in glucose repression (hxk2Δ), or derepression (snf1Δ), and with invertase as a marker enzyme, we show that glucose repression and the osmotic-stress response system regulate glycerol-3-phosphate dehydrogenase synthesis independently. We infer that specific control mechanisms sense the osmotic situation of the cell and induce responses such as the production and retention of glycerol.
Similar content being viewed by others
References
Adler L, Blomberg LA, Nilsson A (1985) J Bacteriol 162:300–306
André L, Hemming A, Adler L (1991) FEBS Lett 286:13–17
Blomberg A, Adler L (1992) Adv Microbiol Physiol 33:145–212
Bradford MM (1976) Anal Biochem 72:248–254
Brown AD (1978) Adv Microbiol Physiol 17:181–242
Brown AD, Mackenzie KF, Singh KK (1986) FEMS Microbiol Rev 39:31–36
Carlson M, Botstein D (1982) Cell 28:145–154
Celenza JL, Carlson M (1986) Science 233:1175–1180
Ciriacy M (1977) Mol Gen Genet 154:213–220
Eck JH van, Prior BA, Brandt EV (1989) J Gen Microbiol 135:3505–3513
Entian K-D, Zimmermann FK (1982) J Bacteriol 151:1113–1118
Entian K-D, Zimmermann FK, Scheel I (1977) Mol Gen Genet 156:99–105
Gancedo C, Serrano R (1989) In: Rose AH, Harrison JS (eds) The yeasts, vol 3 2nd edn. Academic Press, New York, pp 205–259
Gancedo C, Gancedo JM, Sols A (1968) Eur J Biochem 5:165–172
Goldstein A, Lampen JO (1975) Methods Enzymol 42:504–511
Hohmann S, Zimmermann FK (1986) Curr Genet 11:217–225
Hohmann S, Neves MJ, de Koning W, Alijo R, Ramos J, Thevelein JM (1993) Curr Genet 23:281–289
Hubbart EJA, Yang X, Carlson M (1992) Genetics 130:71–80
Laere A van (1989) FEMS Microbiol Rev 63:201–210
Lin ECC, Koch JP, Chused TM, Jorgensen SE (1962) Proc Natl Acad Sci USA 48:2145–2150
May JW, Marshall JH, Sloan J (1982) J Gen Microbiol 128:1763–1766
Nehlin JO, Ronne H (1990) EMBO J 9:2891–2898
Nover L (1991) Heat-shock response. CRC Press, Boca Raton, Florida, USA
Onishi H, Shiromaru Y (1984) FEMS Microbiol Rev 25:175–178
Prior BA, Casaleggio C, Vuuren HJJ van (1977) J Food Protect 40:537–539
Robinson RA, Stokes RH (1959) Electrolyte solutions, 2nd edn. Butterworth, London
Rose M, Albig W, Entian K-D (1991) Eur J Biochem 199:511–518
Sherman F, Fink GR, Hicks JB (1986) Methods in yeast genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sprague GF, Cronan JE (1977) J Bacteriol 129:1335–1342
Thomas BJ, Rothstein R (1989) Cell 56:619–630
Ushio K, Motoko S, Nakata Y (1991) J Ferment Bioeng 71:390–396
Vanhalewyn M, Thevelein JM (1992) Yeast 8 (special issue): S 391
Wiemken A (1990) Ant van Leeuwenhoek 58:209–217
Zamenhof S (1957) Methods Enzymol 3:696–704
Zyl PJ van, Prior BA (1990) Appl Microbiol Biotechnol 33:12–17
Zyl PJ van, Kilian SG, Prior BA (1990) Appl Microbiol Biotechnol 34:231–235
Zyl PJ van, Prior BA, Kilian SG (1991) Appl Microbiol Biotechnol 36:369–374
Author information
Authors and Affiliations
Additional information
Communicated by K. Wolf
Rights and permissions
About this article
Cite this article
Albertyn, J., Hohmann, S. & Prior, B.A. Characterization of the osmotic-stress response inSaccharomyces cerevisiae: osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr Genet 25, 12–18 (1994). https://doi.org/10.1007/BF00712960
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00712960