Abstract
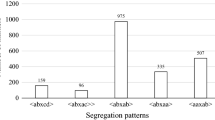
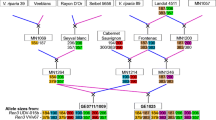
Improved efficacy and durability of powdery mildew resistance can be enhanced via knowledge of the genetics of resistance and susceptibility coupled with the development of high-resolution maps to facilitate the stacking of multiple resistance genes and other desirable traits. We studied the inheritance of powdery mildew (Erysiphe necator) resistance and susceptibility of wild Vitis rupestris B38 and cultivated V. vinifera ‘Chardonnay’, finding evidence for quantitative variation. Molecular markers were identified using genotyping-by-sequencing, resulting in 16,833 single nucleotide polymorphisms (SNPs) based on alignment to the V. vinifera ‘PN40024’ reference genome sequence. With an average density of 36 SNPs/Mbp and uniform coverage of the genome, this 17K set was used to identify 11 SNPs on chromosome 7 associated with a resistance locus from V. rupestris B38 and ten SNPs on chromosome 9 associated with a locus for susceptibility from ‘Chardonnay’ using single marker association and linkage disequilibrium analysis. Linkage maps for V. rupestris B38 (1,146 SNPs) and ‘Chardonnay’ (1,215 SNPs) were constructed and used to corroborate the ‘Chardonnay’ locus named Sen1 (Susceptibility to Erysiphe necator 1), providing the first insight into the genetics of susceptibility to powdery mildew from V. vinifera. The identification of markers associated with a susceptibility locus in a V. vinifera background can be used for negative selection among breeding progenies. This work improves our understanding of the nature of powdery mildew resistance in V. rupestris B38 and ‘Chardonnay’, while applying next-generation sequencing tools to advance grapevine genomics and breeding.





Similar content being viewed by others
References
Adam-Blondon AF, Roux C, Claux D, Butterlin G, Merdinoglu D, This P (2004) Mapping 245 SSR markers on the Vitis vinifera genome: a tool for grape genetics. Theor Appl Genet 109(5):1017–1027
Alleweldt G, Spiegel-Roy P, Reisch B (1991) Grapes (Vitis). In: vol 290. Acta Hort (ISHS), pp 291–330
Barbazuk WB, Bedell JA, Rabinowicz PD (2005) Reduced representation sequencing: a success in maize and a promise for other plant genomes. Bioessays 27(8):839–848
Boubals D (1961) Étude des causes de la résistance des Vitacées à l’Oïdium de la vigne (Uncinula necator (Schw.) Burr.) et de leur mode de transmission héréditaire. Ann Amélior Plant 11:401–500
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23(19):2633–2635
Broman KW, Wu H, Sen S, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890
Cadle-Davidson L, Mahanil S, Gadoury DM, Kozma P, Reisch BI (2011) Natural infection of Run1-positive vines by naïve genotypes of Erysiphe necator. Vitis 50:173–175
Coleman C, Copetti D, Cipriani G, Hoffman S, Kozman P, Kovacs L, Morgante M, Testolin R, Di Gaspero G (2009) The powdery mildew resistance gene REN1 co-segregates with an NBS-LRR gene cluster in two Central Asian grapevines. BMC Genet. 10. doi:10.1186/1471-2156-10-89
Dalbó MA, Ye GN, Weeden NF, Wilcox WF, Reisch BI (2001) Marker-assisted selection for powdery mildew resistance in grapes. J Am Soc Hortic Sci 126(1):83–89
Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, Blaxter ML (2011) Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat Rev Genet 12(7):499–510
Deulvot C, Charrel H, Marty A, Jacquin F, Donnadieu C, Lejeune-Henaut I, Burstin J, Aubert G (2010) Highly-multiplexed SNP genotyping for genetic mapping and germplasm diversity studies in pea. BMC Genomics 11:468
Di Gaspero G, Cattonaro F (2009) Application of genomics to grapevine improvement. Aust J Grape Wine Res 16:122–130
Di Gaspero G, Cipriani G, Adam-Blondon AF, Testolin R (2007) Linkage maps of grapevine displaying the chromosomal locations of 420 microsatellite markers and 82 markers for R-gene candidates. Theor Appl Genet 114(7):1249–1263
Doligez A, Adam-Blondon A, Cipriani G, Di Gaspero G, Laucou V, Merdinoglu D, Meredith C, Riaz S, Roux C, This P (2006) An integrated SSR map of grapevine based on five mapping populations. Theor Appl Genet 113(3):369–382
Eibach R, Zyprian E, Welter LJ, Topfer R (2007) The use of molecular markers for pyramiding resistance genes in grapevine breeding. Vitis 46(3):120–125
Elshire R, Glaubitz J, Sun Q, Poland J, Kawamoto K, Buckler E, Mitchell S (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE. doi:10.1371/journal.pone.0019379
Feechan A, Kabbara S, Dry IB (2010) Mechanisms of powdery mildew resistance in the Vitaceae family. Mol Plant Path doi. doi:10.1111/j.1364-3703.2010.00668.x
Ganal MW, Durstewitz G, Polley A, Bérard A, Buckler ES, Charcosset A, Clarke JD, Graner E-M, Hansen M, Joets J, Le Paslier M-C, McMullen MD, Montalent P, Rose M, Schön C-C, Sun Q, Walter H, Martin OC, Falque M (2011) A large maize (Zea mays L.) SNP genotyping array: Development and germplasm genotyping, and genetic mapping to compare with the B73 reference genome. PLoS ONE. doi:10.1371/journal.pone.0028334
Glaubitz J, Casstevens T, Elshire R, Harriman J, Casstevens T (2012) TASSEL 3.0 genotyping by sequencing (GBS) pipeline documentation. http://www.maizegenetics.net/tassel/docs/TasselPipelineGBS.pdf. Accessed 23 November 2012
IPGRI, UPOV, OIV (1997) Descriptors for grapevine (Vitis spp.). International Union for the Protection of New Varieties of Plants, Geneva, Switzerland/Office International de la Vigne et du Vin, Paris, France/International Plant Genetic Resources Institute, Rome, Italy
Jaillon O, Aury J-M, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, Vezzi A, Legeai F, Hugueney P, Dasilva C, Horner D, Mica E, Jublot D, Poulain J, Bruyère C, Billault A, Segurens B, Gouyvenoux M, Ugarte E, Cattonaro F, Anthouard V, Vico V, Fabbro CD, Alaux M, Gaspero GD, Dumas V, Felice N, Paillard S, Juman I, Moroldo M, Scalabrin S, Canaguier A, Clainche IL, Malacrida G, Durand E, Pesole G, Laucou V, Chatelet P, Merdinoglu D, Delledonne M, Pezzotti M, Lecharny A, Scarpelli C, Artiguenave F, Pè ME, Valle G, Morgante M, Caboche M, Adam-Blondon A-F, Weissenbach J, Quétier F, Wincker P (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449(7161):463–467
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444(7117):323–329
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25(14):1754–1760
Lu F, Lipka AE, Glaubitz J, Elshire R, Cherney JH, Casler MD, Buckler ES, Costish DE (2013) Switchgrass genomic diversity, ploidy, and evolution: novel insights from a network-based SNP discovery protocol. PLoS Genet 9(1):e1003215
Mahanil S, Ramming DW, Cadle-Davidson M, Owens CL, Garris A, Myles S, Cadle-Davidson L (2011) Development of marker sets useful in the early selection of Ren4 powdery mildew resistance and seedlessness for table and raisin grape breeding. Theor Appl Genet. doi:10.1007/s00122-011-1684-7
Moroldo M, Paillard S, Marconi R, Fabrice L, Canaguier A, Cruaud C, De Berardinis V, Guichard C, Brunaud V, Le Clainche I, Scalabrin S, Testolin R, Di Gaspero G, Morgante M, Adam-Blondon A (2008) A physical map of the heterozygous grapevine ‘Cabernet Sauvignon’ allows mapping candidate genes for disease resistance. BMC Plant Biol. doi:10.1186/1471-2229-8-66
Morrell PL, Buckler ES, Ross-Ibarra J (2012) Crop genomics: advances and applications. Nat Rev Genet 13(2):85–96
Myles S, Chia J-M, Hurwitz B, Simon C, Zhong GY, Buckler E, Ware D (2010) Rapid genomic characterization of the genus Vitis. PLoS ONE. e8219. doi:10.1371/journal.pone.0008219
Myles S, Boyko AR, Owens CL, Brown PJ, Grassi F, Aradhya MK, Prins B, Reynolds A, Chia J-M, Ware D, Bustamante CD, Buckler ES (2011) Genetic structure and domestication history of the grape. Proc Natl Acad Sci USA 108(9):3530–3535
Nielsen R, Paul JS, Albrechtsen A, Song YS (2011) Genotype and SNP calling from next-generation sequencing data. Nat Rev Genet 12(6):443–451
Pearson R (1988) Compendium of grape diseases. American Phytopathological Society (APS), Minnesota
Peressotti E, Wiedemann-Merdinoglu S, Delmotte F, Bellin D, Di Gaspero G, Testolin R, Merdinoglu D, Mestre P (2010) Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. doi:10.1186/1471-2229-10-147
Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ (2009) Shades of gray: the world of quantitative disease resistance. Trends Plant Sci 14(1):21–29
Poland JA, Brown PJ, Sorrells ME, Jannink J-L (2012) Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS ONE. doi:10.1371/journal.pone.0032253
Pollard KS, Gilbert HN, Ge Y, Taylor S, Dudoit (2004) S multtest: resampling-based multiple hypothesis testing. R package version 2.12.0
Riaz S, Dangl GS, Edwards KJ, Meredith CP (2004) A microsatellite marker based framework linkage map of Vitis vinifera L. Theor Appl Genet 108(5):864–872
Troggio M, Malacarne G, Coppola G, Segala C, Cartwright DA, Pindo M, Stefanini M, Mank R, Moroldo M, Morgante M, Grando MS, Velasco R (2007) A dense single-nucleotide polymorphism-based genetic linkage map of grapevine (Vitis vinifera L.) anchoring Pinot Noir Bacterial Artificial Chromosome contigs. Genetics 176(4):2637–2650
Van Ooijen JW (2006). JoinMap® 4, Software for the calculation of genetic linkage maps in experimental populations. Kyazma, Wageningen, The Netherlands
Van Tassell CP, Smith TPL, Matukumalli LK, Taylor JF, Schnabel RD, Lawley CT, Haudenschild CD, Moore SS, Warren WC, Sonstegard TS (2008) SNP discovery and allele frequency estimation by deep sequencing of reduced representation libraries. Nat Meth 5(3):247–252
Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, Pruss D, Pindo M, FitzGerald LM, Vezzulli S, Reid J, Malacarne G, Iliev D, Coppola G, Wardell B, Micheletti D, Macalma T, Facci M, Mitchell JT, Perazzolli M, Eldredge G, Gatto P, Oyzerski R, Moretto M, Gutin N, Stefanini M, Chen Y, Segala C, Davenport C, Demattè L, Mraz A, Battilana J, Stormo K, Costa F, Tao Q, Si-Ammour A, Harkins T, Lackey A, Perbost C, Taillon B, Stella A, Solovyev V, Fawcett JA, Sterck L, Vandepoele K, Grando SM, Toppo S, Moser C, Lanchbury J, Bogden R, Skolnick M, Sgaramella V, Bhatnagar SK, Fontana P, Gutin A, Van de Peer Y, Salamini F, Viola R (2007) A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE. doi:10.1371/journal.pone.0001326
Wang N, Fang L, Xin H, Wang L, Li S (2012) Construction of a high-density genetic map for grape using next generation restriction-site associated DNA sequencing. BMC Plant Biol. doi:10.1186/1471-2229-12-148
Ward JA, Bhangoo J, Fernández-Fernández F, Moore P, Swanson JD, Viola R., Velasco R, Bassil N, Weber CA, Sargent DJ (2013) Saturated linkage map construction in Rubus idaeus using genotyping by sequencing and genome-independent imputation. BMC Genomics. doi:10.1186/1471-2164-14-2
Wiedmann R, Smith T, Nonneman D (2008) SNP discovery in swine by reduced representation and high throughput pyrosequencing. BMC Genet. doi:10.1186/1471-2156-9-81
Xie W, Feng Q, Yu H, Huang X, Zhao Q, Xing Y, Yu S, Han B, Zhang Q (2010) Parent-independent genotyping for constructing an ultrahigh-density linkage map based on population sequencing. Proc Natl Acad Sci USA. doi:10.1073/pnas.1005931107
Acknowledgments
We thank Edward Buckler and Qi Sun for support with GBS procedures and genetic analysis; Alex Lipka for statistical advice; and Laura Shannon for her help in R/QTL use and linkage map construction. This research was supported by BecasChile, the USDA Viticulture Consortium—East, the New York Wine & Grape Foundation, and the Lake Erie Regional Grape Processors Fund. Partial funding was also provided by a USDA-National Institute of Food and Agriculture—Specialty Crop Research Initiative Competitive Grant, Award No. 2011-51181-30635.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Töpfer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2013_2202_MOESM1_ESM.tif
Suppl. S1 Number of crossovers per individual as a function of percentage of missing data. Individuals 2, 36, 45, 50, 55, 62, 66, 70, 74 and 76 showing an increased proportion of crossing over were discarded from the linkage analysis (TIFF 81 kb)
122_2013_2202_MOESM2_ESM.pdf
Suppl. S2 The allelic state of each significant SNP in each of the parents and progeny. For each progeny, ‘Chardonnay’ (Ch) and Vitis rupestris B38 (R), each of these biallelic SNPs is coded as dark red or light red, with a blank for missing data. Marker name corresponds with chromosome location in the PN40024 reference genome. S20 markers have not been assigned to a chromosome, and S27 corresponds to unassembled portions of chromosome 7. Locus LD reports the reference chromosome to which the marker was in linkage disequilibrium. S10_16893872 was not in LD with any PN40024 reference chromosome (PDF 36 kb)
122_2013_2202_MOESM3_ESM.pdf
Suppl. S3 Linkage disequilibrium of single SNPs with whole genome SNPs from the corresponding parent, measured as D′. X axis indicates SNP position based on alignment to the physical map (PN40024). Additional information for each of these significant SNPs is provided in Table 2 (PDF 1072 kb)
122_2013_2202_MOESM4_ESM.tif
Suppl. S4 Linkage disequilibrium (LD) analysis based on D′ calculations for a full matrix of selected chromosomes of V. rupestris B38 (A) and ‘Chardonnay’ (B). Chromosomes 7 and 9 contain SNPs associated with powdery mildew resistance and chromosomes 6 and 16 are representative of the pattern observed among the remaining chromosomes. X- and Y-axes indicate SNP positions based on alignment to the physical map (PN40024). Markers with high D′ values are in LD (red to purple) as commonly seen by the within chromosome comparisons. Panel (C) represents an enlargement of the comparison between chromosomes 9 and 16 of ‘Chardonnay’. The arrow shows the position of significant marker S16_11260816. Alignment to the physical map placed this marker in chromosome 16, but LD analysis and the linkage map placed it on chromosome 9. (TIFF 586 kb)
Rights and permissions
About this article
Cite this article
Barba, P., Cadle-Davidson, L., Harriman, J. et al. Grapevine powdery mildew resistance and susceptibility loci identified on a high-resolution SNP map. Theor Appl Genet 127, 73–84 (2014). https://doi.org/10.1007/s00122-013-2202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-013-2202-x