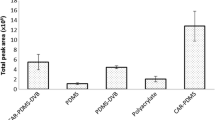
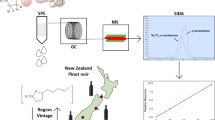
Abstract
The aim of this study was to quantify, in a single analysis, 31 volatile fermentation-derived products that contribute to the aroma of red and white wine. We developed a multi-component method based on headspace solid-phase microextraction coupled with gas chromatography mass spectrometry (HS-SPME-GC-MS). The 31 volatile compounds analysed include ethyl esters, acetates, acids and alcohols. Although these compounds have a range of functional groups, chemical properties, volatilities, affinities for the SPME fibre, and are found in wine at various concentrations, the accuracy of the analysis was achieved with the use of polydeuterated internal standards for stable isotope dilution analyses (SIDA). Nine of the labelled standards were commercially available, while 22 were synthesised. The method was validated by a series of duplicate spiked standard additions to model, white and red wine matrices over the concentration range relevant for each compound in wine. This demonstrated that the appropriate use of SIDA helped to account for matrix effects, for instance potential sources of variation such as the relative response to the MS detector, ionic strength, ethanol content and pH of different wine matrices. The resultant calibration functions had correlation coefficients (R2) ranging from 0.995 to 1.000. Each compound could be quantified at levels below its aroma threshold in wine. Relative standard deviations were all <5%. The method was optimised for the best compromise (over the 31 compounds) of wine dilution factor, level of sodium chloride addition, SPME fibre, SPME temperature, SPME time, GC column and MS conditions. Confirmation of identity was achieved by retention time and peak shape, and measurement of at least three ions for each analyte and internal standard with the MS operating in selected ion monitoring mode to facilitate more precise quantitation with a high sampling rate. The method is a valuable research tool with many relevant applications. A novel method for the combined chiral separation and SIDA quantification of 2- and 3-methylbutanoic acid is also demonstrated.
Similar content being viewed by others
References
Ribereau-Gayon P, Glories Y, Maujean A, Dubourdieu D (2000) The chemistry of wine and stabilization and treatments, vol 2. Wiley, New York, pp 187–206
Rocha SM, Rodrigues F, Coutinho P, Delgadillo I, Coimbra MA (2004) Anal Chim Acta 513:257–262
Kotseridis Y, Baumes R (2000) J Agr Food Chem 48:400–406
Zhou Y, Riesen R, Gilpin CS (1996) J Agr Food Chem 44:818–822
Rapp A (1988) In: Linskens H, Jackson J (eds) Modern methods of plant analysis. Springer, Berlin Heidelberg New York, pp 29–65
Webster DR, Edwards CG, Spayd SE, Peterson JC, Seymour BJ (1993) Am J Enol Viticult 44:275–284
Schreier P (1997) In: In Vino Analytica Scientia, École Européenne de Chimie Analytique. Bordeaux, France, pp17–30
Schreier P (1979) CRC Cr Rev Food Sci 10:59–111
Rapp A, Pretorius PJ (1989) In: Charalambous G (ed) Proc 6th Int Flavour Conf, 5–7 July 1989, Rethymnon, Greece. Elsevier, Amsterdam, pp 1–21
Sejer Pedersen D, Capone DL, Skouroumounis GK, Pollnitz AP, Sefton MA (2003) Anal Bioanal Chem 375:517–522
Rapp A (1998) Nahrung 42:351–363
Drawert F (1974) In: Webb AD (ed) Chemistry of winemaking, 3, ACS Symp Ser 137. ACS, Washington DC, pp 1–10
Rodriguez-Bencomo JJ, Conde JE, Rodriguez-Delgado MA, Garcia-Montelongo F, Perez-Trujillo JP (2002) J Chromatogr A 963:213–223
Etievant PX (1991) In: Maarse H (ed) Volatile compounds in foods and beverages. Marcel Dekker, New York, pp 483–546
Schreier P (1979) CRC Cr Rev Food Sci 10:59–111
Margalith P, Schwartz Y (1970) In: Perlman D (ed) Advances in applied microbiology. Academic, New York, p 35
Guth H (1997) J Agr Food Chem 45:3027–3032
Verstrepen KJ, Derdelinkx G, Dufour J-P, Winderickx J, Thevelein JM, Pretorius IS, Delvaux FR (2003) J Biosci Bioeng 96:110–118
Mallalouchos A, Komaitis M, Koutinas A, Kanellaki M (2002) J Agr Food Chem 50:3840–3848
Lambrechts MG, Pretorius IS (2000) S Afr J Enol Viticult 21:97–129
Ortega C, López R, Cacho J, Ferreira V (2001) J Chromatogr A 923:205–214
Suomalainen H (1981) J I Brewing 87:296–300
Nykänen L (1986) Am J Enol Viticult 37:84–96
Ferreira V, Lopez R, Cacho JF (2000) J Sci Food Agric 80:1659–1667
Suomalainen H, Lehtonen M (1979) J I Brewing 85:149–156
Killian E, Ough CS (1979) Am J Enol Viticult 30:301–305
Antonelli A, Castellari L, Zambonelli C, Carnacini A (1999) J Agr Food Chem 47:1139–1144
Alvarez MA, Garcia E (1984) Microbiol Esp 37:37–45
Aragon P, Atienza J, Climent MD (1998) Am J Enol Viticult 49:211–216
Patel S, Shibamoto T (2003) J Food Comp Anal 16:469–476
Fleet GH (1990) J Wine Res 1:211–223
Lema C, Garcia-Jares C, Orriols I, Angulo L (1996) Am J Enol Viticult 47:206–216
García MA, Oliva J, Barba A, Cámara MÁ, Díaz-Plaza EM (2004) J Agr Food Chem 52:1241–1247
Giudici P, Romano P, Zambonelli C (1990) Can J Microbiol 36:61–64
Salo P (1970) J Food Sci 35:95–99
Meilgaard MC (1975) MBAA Tech Q 12:151–168
Simpson RF (1979) Food Technol Aust 31:516–522
Amerine MA, Roessler EB (1976) Wines—their sensory evaluation. WH Freeman, New York, pp 72–77
Watts VA, Butzke CE (2003) J Sci Food Agric 83:1143–1149
Bonino M, Schellino R, Rizzi C, Aigotti R, Delfini C, Baiocchi C (2003) Food Chem 80:125–133
Boido E, Lloret A, Medina K, Fariña L, Carrau F, Versini G, Dellacassa E (2003) J Agr Food Chem 51:5408–5413
Miklósy E, Kerényi Z (2004) Anal Chim Acta 513:177–181
Spranger MI, Clímaco MC, Sun B, Eiriz N, Fortunato C, Nunes A, Leandro MC, Avelar ML, Belchior AP (2004) Anal Chim Acta 513:151–161
Castro R, Natera R, Benitez P, Barroso CG (2004) Anal Chim Acta 513:141–150
Ferreira V, Rapp A, Cacho JF, Hastrich H, Yavas I (1993) J Agr Food Chem 41:1413–1420
Yang X, Peppard T (1994) J Agr Food Chem 42:1925–1930
Escudero A, Gogorza B, Melús MA, Ortín N, Cacho J, Ferreira V (2004) J Agr Food Chem 52:3516–3524
Escalona H, Birkmyre L, Piggott JR, Paterson A (2002) Anal Chim Acta 458:45–54
Peinado RA, Moreno JJ, Oretga JM, Mauricio JC (2003) J Agr Food Chem 51:6198–6203
Rogerson FSS, De Freitas VAP (2002) J Food Sci 67:1564–156
Shao Y, Marriott P (2003) Anal Bioanal Chem 375:635–642
Rowan DD, Lane HP, Allen JM, Fielder S, Hunt MB (1996) J Agr Food Chem 44:3276–3285
Pollnitz AP, Jones GP, Sefton MA (1999) J Chromatogr A 857:239–246
Urruty L, Montury M (1996) J Agr Food Chem 44:3871–3877
Verhoeven H, Beuerle T, Schwab W (1997) Chromatographia 46:63–66
Zhang Z, Pawliszyn J (1993) Anal Chem 65:1843–1852
Hawthorne SB, Miller DJ, Pawliszyn J, Arthur CL (1992) J Chromatogr 603:185–191
Arthur CL, Pawliszyn J (1990) Anal Chem 62:2145–2148
Hayasaka Y, MacNamara K, Baldock GA, Taylor RL, Pollnitz AP (2003) Anal Bioanal Chem 375:948–955
Coulter A, Robinson E, Cowey G, Francis IL, Lattey K, Capone D, Gishen M, Godden, P (2003) In: Bell SM, de Garis KA, Dundon CG, Hamilton RP, Partridge SJ, Wall GS (eds) Grapegrowing at the edge; managing the wine business; impacts on wine flavour: proceedings of a seminar. Barossa Convention Centre, Tanunda, S.A., 10–11 July 2003. Australian Society of Viticulture and Oenology, Adelaide, Australia, pp 41–50
Smyth H, Cozzolino D, Francis IL (2003) In: Bell SM, de Garis KA, Dundon CG, Hamilton RP, Partridge SJ, Wall GS (eds) Grapegrowing at the edge; managing the wine business; impacts on wine flavour: proceedings of a seminar. Barossa Convention Centre, Tanunda, S.A., 10–11 July 2003. Australian Society of Viticulture and Oenology, Adelaide, Australia, pp 56–58
Pollnitz A, Eglinton J, Siebert T, Smyth H, Henschke P, Parker M, Francis L, Cozzolino D, Herderich M (2004) Australian Vignerons (July/Aug) 17–21
Acknowledgments
Kate Howell is acknowledged for her work in the preliminary development and validation of methodology for the analysis of the acetates at the Australian Wine Research Institute (AWRI). We thank Gordon Elsey of the AWRI for his expertise with NMR. Jose Newton is thanked for her technical assistance distilling labelled standards. We thank Peter Winterhalter (Institute of Food Chemistry, Technical University of Braunschweig) and Sakkie Pretorius, Peter Høj and Leigh Francis of the AWRI for valued discussions and encouragement. This project is supported by Australia’s grapegrowers and winemakers through their investment body the Grape and Wine Research and Development Corporation, with matching funds from the Australian Government, under projects AWR2 and AWR6, and by the Commonwealth Cooperative Research Centres Program of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siebert, T.E., Smyth, H.E., Capone, D.L. et al. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal Bioanal Chem 381, 937–947 (2005). https://doi.org/10.1007/s00216-004-2992-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2992-4