Abstract
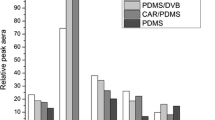
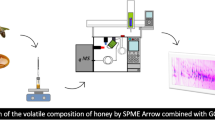
Volatile compounds from 40 honey samples of different botanic origin were analyzed by SPME followed by GC–MS. In order to obtain complementary data for an overall characterization of honey aroma, two different SPME fiber coatings (polyacrylate and carboxen/polydimethylsiloxane) were employed. The use of both fibers with a single chromatographic column afforded the identification or characterization, based on GC retention and mass spectral data, of a total of 193 volatile components. A total of 166 honey volatiles were characterized (146 identified) from CAR/PDMS data, this fiber being the most appropriate for isolation of low molecular weight compounds. Polyacrylate fiber was better for extraction of polar semivolatiles, allowing to identify 120 compounds and to characterize 132. Besides typical nectar components such as limonene, linalool, etc., different compounds from fermentation (ethanol and 2,3-butanediol), processing (furan derivatives), hive treatment (thymol), etc. were detected. Although many volatiles were common to most honey samples analyzed, other seemed to be characteristic of certain honey types.






Similar content being viewed by others
References
Bicchi C, Belliardo F, Frattini C (1983) J Apic Res 22:130–136
Rowland CY, Blackman AJ, D’Arcy BR, Rintoul GB (1995) J Agric Food Chem 43:753–763
D’Arcy BR, Rintoul GB, Rowland CY, Blackman AJ (1997) J Agric Food Chem 45:1834–1843
Tan ST, Holland PT, Wilkins AL, Molan PC (1988) J Agric Food Chem 36:453–460
Bonaga G, Giumanini AG (1986) J Apic Res 25:113–120
Alissandrakis E, Tarantilis PA, Harizanis PC, Polissiou M (2004) J Sci Food Agric 85:91–97
Bouseta A, Collin S (1995) J Agric Food Chem 43:1890–1897
Bouseta A, Collin S, Dufour JP (1992) J Apic Res 31:96–109
Overton SV, Manura JJ (1994) Am Lab 26:45, 47–53
Radovic BS, Careri M, Mangia A, Musci M, Gerboles M, Anklam E (2001) Food Chem 72:511–520
Soria AC, Martínez-Castro I, Sanz J (2007) J Chromatogr A 1157:430–436
Arthur CL, Pawliszyn J (1990) Anal Chem 62:2145–2148
Pillonel L, Bosset JO, Tabacchi R (2002) Lebensm-Wiss u-Technol 35:1–14
Guidotti M, Vitali M (1998) Ind Aliment 37:351–353, 356
Verzera A, Campisi S, Zappalà M, Bonaccorsi I (2001) Am Lab News 7:18–21
Pérez RA, Sánchez-Brunete C, Calvo RM, Tadeo JL (2002) J Agric Food Chem 50:2633–2637
Soria AC, Martínez-Castro I, Sanz J (2003) J Sep Sci 26:793–801
Piasenzotto L, Gracco L, Conte L (2003) J Sci Food Agric 83:1037–1044
Bentivenga G, D’Auria M, Fedeli P, Mauriello G, Racioppi R (2004) Int J Food Sci Technol 39:1079–1086
Soria AC, González M, de Lorenzo C, Martínez-Castro I, Sanz J (2004) Food Chem 85:121–130
Ampuero S, Bogdanov S, Bosset J (2004) Eur Food Res Technol 218:198–207
Soria AC, González M, de Lorenzo C, Martínez-Castro I, Sanz J (2005) J Sci Food Agric 85:817–824
Alissandrakis E, Kibaris AC, Tarantilis PA, Harizanis PC, Polissiou M (2005) J Sci Food Agric 85:1444–1452
de la Fuente E, Martínez-Castro I, Sanz J (2005) J Sep Sci 28:1093–1100
Baroni MV, Nores ML, Díaz MP, Chiabrando GA, Fassano JP, Costa C, Wunderlin DA (2006) J Agric Food Chem 54:7235–7241
Cuevas-Glory LF, Pino JA, Santiago LS, Sauri-Duch E (2007) Food Chem 103:1032–1043
Odeh I, Abu-Lafi S, Dewik H, Al-Najjar I, Imam A, Dembitsky VM, Hanus LO (2007) Food Chem 101:1393–1397
Mannas D, Altug T (2007) Int J Food Sci Technol 42:133–138
Alissandrakis E, Tarantilis PA, Harizanis PC, Polissiou M (2007) Food Chem 100:396–404
McLafferty FW, Stauffe DB (1989) The Wiley/NBS registry of mass spectral data. Wiley, New York
Soria AC, Martínez-Castro I, de Lorenzo C, Sanz J (2008) Food Chem 107:439–443
de la Fuente E, Valencia-Barrera RM, Martínez-Castro I, Sanz J (2007) Food Chem 103:1176–1180
McReynolds WO (1966) Gas chromatographic retention data. Preston Technical Abstracts Company, Evanston
Bianchi F, Careri M, Mangia A, Musci M (2007) J Sep Sci 30:563–572
http://www.pherobase.com/database/floral-compounds/floral-taxa-compounds-index.php
Shimoda M, Wu Y, Osajima Y (1996) J Agric Food Chem 44:3913–3918
Jennings W, Shibamoto T (1980) Qualitative analysis of flavor and fragrante volatiles by glass capillary gas chromatography. Academic Press, New York
Bianchi F, Careri M, Musci M (2005) Food Chem 89:527–532
Davies NW (1990) J Chromatogr A 503:1–24
Guyot C, Scheirman V, Collin S (1999) Food Chem 64:3–11
Castro-Vázquez L, Pérez-Coello MS, Cabezudo MD (2003) Chromatographia 57:227–233
Cajka T, Hajslová J, Cochran J, Holadová K, Klimánková E (2007) J Sep Sci 30:534–546
Njoroge SM, Koaze H, Mwaniki M, Tu NTM, Sawamura M (2005) Flavour Fragr J 20:74–79
Bouseta A, Scheirman V, Collin S (1996) J Food Sci 61:683–687
Guyot-Declerck C, Renson S, Bouseta A, Collin S (2002) Food Chem 79:453–459
Campos G, Nappi GU, Raslan DS, Augusti R (2000) Cienc Technol Aliment 20:18–22
de la Fuente E, Sanz ML, Martínez-Castro I, Sanz J, Ruiz-Matute AI (2007) Food Chem 105:84–93
Tan ST, Wilkins AL, Holland PT, McGhie TK (1989) J Agric Food Chem 37:1217–1221
Graddon AD, Morrison JD, Smith JF (1979) J Agric Food Chem 27:832–837
Tsuneya T, Shibai T, Yoshioka A, Shiga M (1974) Koryo 109:29–35
Blank I, Fischer KH, Grosch W (1989) Z Lebensm Unters Forsch 189:426–433
Guyot C, Bouseta A, Scheirman V, Collin S (1998) J Agric Food Chem 46:625–633
Häusler M, Montag A (1990) Dtsch Lebensm-Rundsch 86:171–174
Ferber CEM, Nursten HE (1977) J Sci Food Agric 28:511–518
Viñas P, Soler-Romera MJ, Hernández-Córdoba M (2006) Talanta 69:1063–1067
Acknowledgments
This work has been funded by projects CTQ2006-14993/BQU and ANALISYC-S-505/AGR-0312 supported by CYCIT and Comunidad de Madrid, respectively. A.C.S. also thanks CSIC and the EU for a postdoctoral I3P contract.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Volatile compounds fractionated by SPME and identified or characterized by GC–MS in each of the honey samples under study appear listed in table 2 (for PA fiber) and table 3 (for CAR/PDMS), grouped by their honey type (rosemary, eucalyptus, citrus, thyme, acacia, rhododendron, chestnut, dandelion, lavender, heather, Teide broom, multiflower and honeydew). The characteristic presence of a volatile compound in a single source was indicated in these tables according to the following criterion: “xx” for a clear presence in most of the analyzed honey samples from the same source and “x” for compounds detected in trace amounts and/or only present in a few samples of that source. “x” was also used for volatiles detected in honey sources for which only one sample was available. This material is available free of charge via the Internet in the online version of this paper only.
217_2008_966_MOESM1_ESM.doc
Supplementary Table 2. Volatile compounds fractionated by SPME using a CAR/PDMS fiber coating in honeys from different source (DOC 504 kb)
217_2008_966_MOESM2_ESM.doc
Supplementary Table 3. Volatile compounds fractionated by SPME using a PA fiber coating in honeys from different source (DOC 401 kb)
Rights and permissions
About this article
Cite this article
Soria, A.C., Sanz, J. & Martínez-Castro, I. SPME followed by GC–MS: a powerful technique for qualitative analysis of honey volatiles. Eur Food Res Technol 228, 579–590 (2009). https://doi.org/10.1007/s00217-008-0966-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-008-0966-z