Abstract
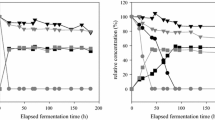
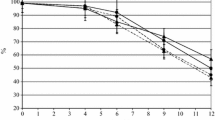
Herein, we isolate and characterize wine yeasts with the ability to reduce volatile acidity of wines using a refermentation process, which consists in mixing the acidic wine with freshly crushed grapes or musts or, alternatively, in the incubation with the residual marc. From a set of 135 yeast isolates, four strains revealed the ability to use glucose and acetic acid simultaneously. Three of them were identified as Saccharomyces cerevisiae and one as Lachancea thermotolerans. Among nine commercial S. cerevisiae strains, strains S26, S29, and S30 display similar glucose and acetic acid initial simultaneous consumption pattern and were assessed in refermentation assays. In a medium containing an acidic wine with high glucose–low ethanol concentrations, under low oxygen availability, strain S29 is the most efficient one, whereas L. thermotolerans 44C is able to decrease significantly acetic acid similar to the control strain Zygosaccharomyces bailii ISA 1307 but only under aerobic conditions. Conversely, for low glucose–high ethanol concentrations, under aerobic conditions, S26 is the most efficient acid-degrading strain, while under limited-aerobic conditions, all the S. cerevisiae strains studied display acetic acid degradation efficiencies identical to Z. bailii. Moreover, S26 strain also reveals capacity to decrease volatile acidity of wines. Together, the S. cerevisiae strains characterized herein appear promising for the oenological removal of volatile acidity of acidic wines.


Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Arneborg N, Jespersen L, Jakobsen M (2000) Individual cells of Saccharomyces cerevisiae and Zygosaccharomyces bailii exhibit different short-term intracellular pH responses to acetic acid. Arch Microbiol 174(1–2):125–128
Boulton RB, Singleton VL, Bisson LF, Kunkee RE (1998) Yeasts and biochemistry of ethanol fermentation, principles and practices of winemaking, 1st edn. Springer, New York, pp 102–192
Casal M, Leão C (1995) Utilization of short-chain monocarboxylic acids by the yeast Torulaspora delbrueckii: specificity of the transport systems and their regulation. Biochim Biophys Acta 1267:122–130
Casal M, Cardoso H, Leão C (1998) Effects of ethanol and other alkanols on transport of acetic acid in Saccharomyces cerevisiae. Appl Environ Microbiol 64(2):665–668
Dos Santos MM, Gombert AK, Christensen B, Olsson L, Nielsen J (2003) Identification of in vivo enzyme activities in the cometabolism of glucose and acetate by Saccharomyces cerevisiae by using 13C-labeled substrates. Eukaryot Cell 2(3):599–608
Du Toit W (2002) Winemaking with rotten grapes: It can be a headache. Wynboer, a technical guide for wine producers. http://www.wynboer.co.za/recentarticles/1202rotten.php3. Accessed 7 June 2007.
Englinton JM, Heinrich AJ, Pollnitz AP, Langridge P, Henschke, PA, Lopes MB (2002) Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast 19(4):295–301
Gerós H, Cássio F, Leão C (2000) Utilization and transport of acetic acid in Dekkera anomala and their implications on the survival of the yeast in acidic environments. J Food Prot 63:96–101
Hansen HE, Nissen P, Sommer P, Nielsen JC, Arneborg N (2001) The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J Appl Microbiol 91:541–547
Jost P, Piendl A (1975) Technological influences on the formation of acetate during fermentation. J Am Soc Brew Chem 34:31–37
Kurtzman CP (2003) Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res 4(3):233–224
Kurtzman CP, Robnett CJ (2003) Phylogenetic relationships among yeasts of the ‘Saccharomyces complex’ determined from multigene sequence analyses. FEMS Yeast Res 3(4):417–432
Lachance MA (1998) The genus Kluyveromyces. In: Kurtzman CP, Fell JW (eds) The yeasts, a taxonomic study. 4th edn. Elsevier, Amsterdam, pp 230–250
Leão C, Van Uden N (1986) Transport of lactate and other short-chain monocarboxylates in the yeast Candida utilis. Appl Microbiol Biotechnol 23:389–393
Lopez V, Querol A, Ramon D, Fernandez-Espinar MT (2001) A simplified procedure to analyse mitochondrial DNA from industrial yeasts. Int J Food Microbiol 68(1–2):75–81
Mohammad JT, Niklasson C, Liden G (1997) Acetic acid friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem Eng Science 52(15):2653–2659
O’Donnell K (1993) “Fusarium and its near relatives”. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CABI, Wallingford, pp 225–233
Pérez MA, Gallego FJ, Martinez I, Hidalgo P (2001) Detection, distribution and selection of microsatellites (SSRs) in the genome of the yeast Saccharomyces cerevisiae as molecular markers. Lett Appl Microbiol 33(6):461–466
Remize F, Roustan JL, Sablayrolles JM, Barre P, Dequin S (1999) Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and stimulation of fermentation rate in stationary phase. Appl Environ Microbiol 65:143–149
Remize F, Andrieu E, Dequin S (2000) Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: role of the cytosolic Mg+ and mitochondrial K+ acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl Environ Microbiol 66(8):3151–3159
Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A (2000) The microbiology of wine and vinifications. Handbook of enology, vol. 1, 1st edn. Wiley, Chichester
Rodrigues F (1998) Estudos sobre a Regulação do Metabolismo Intracelular de Ácido Acético na Levedura Zygosaccharomyces bailli ISA 1307. Tese de Mestrado, Universidade do Minho, Braga
Saint-Prix F, Bönquist L, Dequin S (2004) Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: the NADP +-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology 150:2209–2220
Sousa MJ, Rodrigues F, Côrte-Real M, Leão C (1998) Mechanisms underlying the transport and intracellular metabolism of acetic acid in the presence of glucose in the yeast Zygosaccharomyces bailii. Microbiology 144:665–670
Schüller HJ (2003) Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr Genet 43(3):139–160
Schuller D, Côrte-Real M, Leão C (2000) A differential medium for the enumeration of the spoilage yeast Zygosaccharomyces bailii in wine. J Food Prot 63(11):1570–1575
Schuller D, Valero E, Dequin S, Casal M (2004) Survey of molecular methods for the typing of wine yeast strains. FEMS Microbiol Lett 231(1):19–26
Van Uden N (1967) Transport-limited fermentation and growth of Saccharomyces cerevisiae and its competitive inhibition. Arch Mikrobiol 58:155–168
Acknowledgements
This study was supported by the program POCI 2010 (FEDER/FCT, POCI/AGR/56102/2004, Fundação para a Ciência e Tecnologia).
Work conducted in Dra. Arlete Mendes Faia laboratory was funded by CGB–IBB and by the project PTDC/AGRALI/71460/2006, Fundação para a Ciência e Tecnologia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilela-Moura, A., Schuller, D., Mendes-Faia, A. et al. Reduction of volatile acidity of wines by selected yeast strains. Appl Microbiol Biotechnol 80, 881–890 (2008). https://doi.org/10.1007/s00253-008-1616-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1616-x