-
PDF
- Split View
-
Views
-
Cite
Cite
Mingyi Jiang, Jianhua Zhang, Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings, Plant and Cell Physiology, Volume 42, Issue 11, 15 November 2001, Pages 1265–1273, https://doi.org/10.1093/pcp/pce162
Close - Share Icon Share
Abstract
Leaves of maize (Zea mays L.) seedlings were supplied with different concentrations of abscisic acid (ABA). Its effects on the levels of superoxide radical (O2–), hydrogen peroxide (H2O2) and the content of catalytic Fe, the activities of several antioxidative enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR), the contents of several non-enzymatic antioxidants such as ascorbate (ASC), reduced glutathione (GSH), α-tocopherol (α-TOC) and carotenoid (CAR), and the degrees of the oxidative damage to the membrane lipids and proteins were examined. Treatment with 10 and 100 µM ABA significantly increased the levels of O2– and H2O2, followed by an increase in activities of SOD, CAT, APX and GR, and the contents of ASC, GSH, α-TOC and CAR in a dose- and time-dependent pattern in leaves of maize seedlings. An oxidative damage expressed as lipid peroxidation, protein oxidation, and plasma membrane leakage did not occur except for a slight increase with 100 µM ABA treatment for 24 h. Treatment with 1,000 µM ABA led to a more abundant generation of O2– and H2O2 and a significant increase in the content of catalytic Fe, which is critical for H2O2-dependent hydroxyl radical production. The activities of these antioxidative enzymes and the contents of α-TOC and CAR were still maintained at a higher level, but no longer further enhanced when compared with the treatment of 100 µM ABA. The contents of ASC and GSH had no changes in leaves treated with 1,000 µM ABA. These results indicate that treatment with low concentrations of ABA (10 to 100 µM) induced an antioxidative defence response against oxidative damage, but a high concentration of ABA (1,000 µM) induced an excessive generation of AOS and led to an oxidative damage in plant cells.
(Received April 16, 2001; Accepted September 8, 2001).
Introduction
The phytohormone abscisic acid (ABA) is a sesquiterpenoid and synthesized from xanthophylls (Taylor et al. 2000). ABA plays prominent roles in various physiological processes including induction of seed dormancy and adaptive responses to environmental stresses (Nambara et al. 1998). ABA is involved in the signal transduction pathway regulating many genes that are expressed at specific developmental stages or as a result of an environmental stress (Shinozaki and Yamaguchi-Shinozaki 1997, Siddiqui et al. 1998). ABA accumulates in plant tissues subjected to drought, salt, desiccation, and cold, and is thought to act as a signal for the initiation of acclimation to these stresses (Chandler and Robertson 1994, Shinozaki and Yamaguchi-Shinozaki 1997, Siddiqui et al. 1998, Hare et al. 1999). However, details about how the ABA signal is transduced into a physiological response remain unknown.
Circumstantial evidence indicates that one model of ABA action may be related to oxidative stress in plant cells. It has been shown that ABA can cause an increased generation of H2O2 (Guan et al. 2000, Pei et al. 2000), induce the expression of antioxidant genes encoding Cu, Zn-superoxide dismutase (Cu, Zn-SOD) (Sakamoto et al. 1995, Guan and Scandalios 1998a, Kaminaka et al. 1999), Mn-SOD (Zhu and Scandalios 1994, Bueno et al. 1998, Kaminaka et al. 1999) and Fe-SOD (Kaminaka et al. 1999), and catalase (CAT) (Williamson and Scandalios 1992, Anderson et al. 1994, Guan and Scandalios 1998b, Guan et al. 2000), and increase the activities of antioxidative enzymes such as SOD, CAT, guaiacol peroxidase (GPX), ascorbate peroxidase (APX) and glutathione reductase (GR) in plant tissues (Anderson et al. 1994, Prasad et al. 1994a, Bueno et al. 1998, Gong et al. 1998, Bellaire et al. 2000). In addition, ABA can cause the increase of lipid peroxidation expressed as malondialdehyde (MDA) production in plant cells (Bueno et al. 1998). Therefore, ABA leads to an oxidative stress in plant cells.
Although it has been shown that ABA can result in an oxidative stress, an enhancement in the capacity of oxidative stress tolerance may imply that plants need to mobilize the whole antioxidant defence systems including enzymatic and non-enzymatic constituents to resist oxidative damage in stressed plant tissues, rather than a few enzymes or metabolites. Meanwhile, since ABA causes oxidative stress in plants, it is possible that an oxidative damage might take place in plants exposed to the treatment of high concentrations of ABA. Different concentrations of ABA from 1 to 1,000 µM have been used to different plant tissues and have been shown to be able to induce gene expression and protein synthesis involved in antioxidative defences (Anderson et al. 1994, Prasad et al. 1994a, Zhu and Scandalios 1994, Bueno et al. 1998, Gong et al. 1998, Guan and Scandalios 1998a, Guan and Scandalios 1998b, Kaminaka et al. 1999, Bellaire et al. 2000, Guan et al. 2000). It is essential to assess the effects of high concentrations of ABA treatments on oxidative damage to important molecules such as lipids and proteins. Furthermore, in addition to H2O2, it is not clear whether other active oxygen species (AOS), such as O2– and •OH, are involved in oxidative stress in plants caused by ABA.
In this study, the effects of different concentrations of ABA on the levels of AOS including O2– and H2O2, the content of catalytic Fe, which represents the fraction of Fe bound to low molecular mass chelating agents or loosely bound to certain proteins in a tissue that is active in the generation of •OH and possibly other AOS through Fenton chemistry (Evans and Halliwell 1994, Moran et al. 1994, Gogorcena et al. 1997), the activities of antioxidant enzymes such as SOD, CAT, APX and GR, the contents of non-enzymatic antioxidants ascorbate (ASC), GSH, α-tocopherol (α-TOC) and carotenoid (CAR), and the degrees of oxidative damage to membrane lipids and proteins were systematically studied in details. The objective of the present study was to find out a possible relationship between ABA, AOS, antioxidative defences, and oxidative damage in maize plants.
Results
Effect of ABA on levels of AOS and the content of catalytic Fe
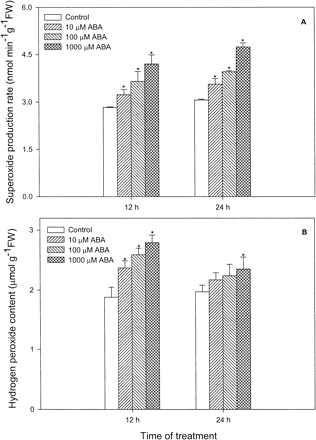
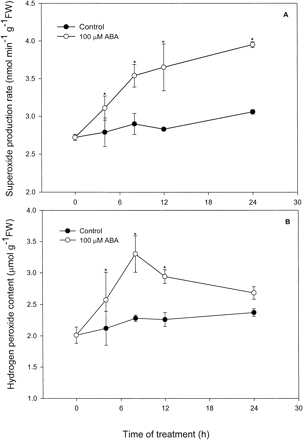
Effects of different concentrations of ABA on levels of O2– and H2O2 in leaves of maize seedlings were investigated and a dose-dependent manner was observed (Fig. 1). Treatment with 10 µM, 100 µM and 1,000 µM ABA caused a significant increase in levels of O2– (Fig. 1A)and H2O2 (Fig. 1B) compared with the control. At 12 h, O2– levels were increased by 14%, 29% and 49%, and H2O2 levels increased by 26%, 38% and 48%, respectively, for the three concentrations of ABA used. At 24 h, levels of O2– remained almost unchanged or a slight increase compared with that of 12 h treatment, but levels of H2O2 decreased significantly, and returned almost to the level of control. The time course of ABA’s action on the levels of O2– and H2O2 showed that treatment of 100 µM ABA significantly enhanced the levels of O2– and H2O2 within the first 4 h of treatment (Fig. 2). For O2–, treatment with ABA caused a rapid increase at the first 8 h, and then remained almost unchanged within 8–24 h (Fig. 2A). For H2O2, treatment with ABA at the first 8 h reached a maximum value, and then decreased significantly, back to the level of control at 24 h (Fig. 2B).
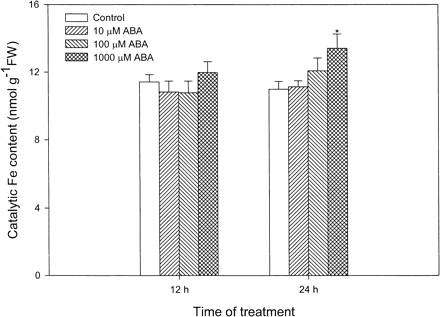
In order to investigate whether other AOS, especially the highly reactive and destructive •OH radicals, are involved in the oxidative stress caused by ABA, we determined the content of catalytic Fe, which is critical for H2O2-dependent •OH production. Fig. 3 shows that only treatment with 1,000 µM ABA for 24 h led to a significant increase in the content of catalytic Fe, and the other treatments did not affect the content of catalytic Fe when compared to the control.
Effect of ABA on activities of antioxidative enzymes
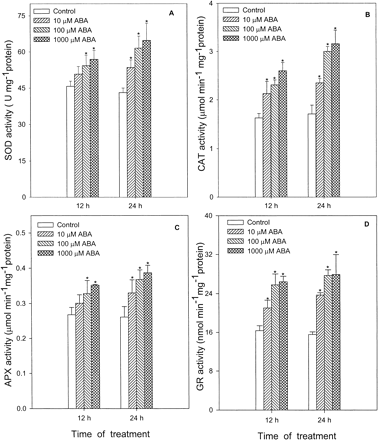
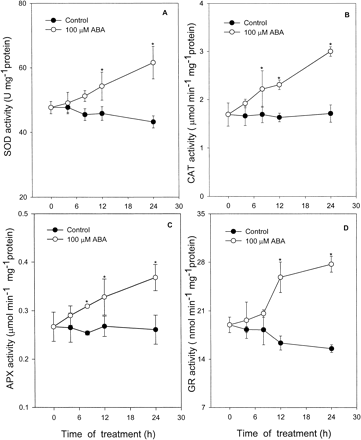
Fig. 4 shows the effects of different concentrations of ABA on several antioxidative enzymes such as SOD (Fig. 4A), the enzyme for catalyzing the dismutation of O2– to O2 and H2O2, CAT (Fig. 4B), the enzyme mainly responsible for elimilating H2O2 in the peroxisomes, and APX (Fig. 4C) and GR (Fig. 4D), the two key enzymes of the Halliwell-Asada pathway for the removal of H2O2 in the chloroplasts. Treatment with 10 µM, 100 µM and 1,000 µM ABA caused a continuous increase in the activities of the four antioxidative enzymes in a dose-dependent pattern within 24 h. At 24 h, leaves treated with 10 µM, 100 µM and 1,000 µM ABA enhanced the activities of SOD by 24%, 43% and 50%, respectively, when compared with the control (Fig. 4A); CAT by 37%, 75% and 85% (Fig. 4B); APX by 26%, 42% and 48% (Fig. 4C); and GR by 52%, 78% and 79% (Fig. 4D). The time course of ABA-enhanced antioxidative enzyme activities showed that significant changes occurred only after 4 h of treatment (Fig. 5). For CAT (Fig. 5B) and APX (Fig. 5C), a significant increase in its activities occurred at 4 to 8 h treatment of 100 µM ABA, compared with the control leaves; and for SOD (Fig. 5A) and GR (Fig. 5D), it occurred at 8 to 12 h.
Effect of ABA on non-enzymatic antioxidants
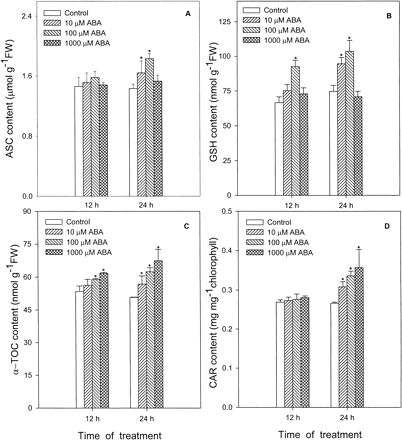
Treatment with ABA also affected the contents of ASC (Fig. 6A) and GSH (Fig. 6B), two main water-soluble antioxidants, and α-TOC (Fig. 6C) and CAR (Fig. 6D), two main lipid-soluble antioxidants in leaves of maize seedlings. No significant changes were observed in the contents of antioxidants in leaves treated with 10 to 1,000 µM ABA within the first 12 h except for 100 µM ABA on the GSH content and 100 to 1,000 µM ABA on the α-TOC content. At 24 h, treatment of leaves with 10 to 1,000 µM ABA significantly enhanced the contents of CAR and α-TOC in a dose-dependent manner. For ASC and GSH, however, a significant increase only occurred in leaves treated with 10 to 100 µM ABA. Treatment with 1,000 µM ABA did not affect the contents of ASC and GSH.
Oxidative damage to biomolecules and membranes
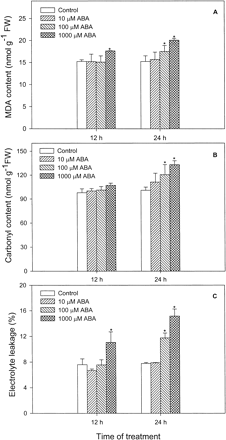
The oxidation of lipids and proteins, which may occur in animal and plant cells that are exposed to adverse conditions, is a reliable indication of uncontrolled AOS production and hence of oxidative stress. The effects of ABA on the levels of lipid peroxides (thiobarbituric acid test) and oxidized proteins (total carbonyl groups) and plasma membrane leakage (electrolyte leakage %) in leaves of maize seedlings are shown in Fig. 7. A marked oxidative damage was observed in leaves treated with 1,000 µM ABA within the first 12 h, and 100 to 1,000 µM ABA at 24 h. No major changes were observed in the levels of MDA (Fig. 7A), C=O groups (Fig. 7B) and electrolyte leakage (Fig. 7C) in leaves incubated with 10 µM ABA for 24 h.
Discussion
It has been shown that ABA can cause an increased production of H2O2, induce the expression of some antioxidant genes and enhance the activities of antioxidative enzymes such as SOD, CAT, GPX, APX and GR in plants (Bueno et al. 1998, Gong et al. 1998, Guan and Scandalios 1998a, Guan and Scandalios 1998b, Kaminaka et al. 1999, Bellaire et al. 2000, Guan et al. 2000). These suggest that ABA can result in an oxidative stress in plants. Our results not only have shown that ABA induced an increase in the content of H2O2 (Fig. 1) and the activities of SOD, CAT, APX and GR (Fig. 4) in leaves of maize seedlings, but also further indicated that treatment of ABA led to an increase in the level of O2– (Fig. 1) and the content of catalytic Fe (Fig. 3), which is a stringent requirement for H2O2-dependent •OH production, and the contents of important antioxidative metabolites such as ASC, GSH, α-TOC and CAR (Fig. 6) in leaves of maize seedlings. Meanwhile, an obvious oxidative damage to lipids and proteins was observed in leaves that were treated with high concentrations of ABA (Fig. 7). These results further support that ABA results in an oxidative stress in plants.
AOS are generally believed as the by-products of oxygen metabolism and can have adverse effects such as lipid peroxidation, denaturation of proteins, and mutation of DNA, all considered as cellular oxidative damage. Abundant evidence, however, has shown that AOS, especially H2O2 and O2–, are involved in cellular signaling process as secondary messengers to induce a number of genes and proteins involved in stress defences, including CAT, GPX, GP, GR, GST, APX, SOD, and pathogenesis-related (PR) protein (Levine et al. 1994, Prasad et al. 1994a, Prasad et al. 1994b, Rao et al. 1997, Dat et al. 1998, Kawano et al. 1998, Morita et al. 1999, Bellaire et al. 2000, Dat et al. 2000, Guan et al. 2000). Although ABA and AOS are thought to act as secondary messengers for the induction of antioxidant defences in stressed plants, the relationship between ABA and AOS in stress signal transduction cascades is still unclear. One possible model is that ABA-mediated metabolic changes might lead to an increase in endogenous AOS levels, which in turn induce antioxidative gene expression (Guan and Scandalios 1998a, Guan and Scandalios 1998b, Guan et al. 2000). Our results showed that a significant increase in levels of O2– and H2O2 occurred within 4 h of ABA treatment (Fig. 2), and the increase in activities of antioxidative enzymes took place at 4–8 h (Fig. 5), followed by the contents of non-enzymatic antioxidants in leaves of maize seedlings (Fig. 6); meanwhile, the enhancement in the activities of these antioxidative enzymes and the contents of these metabolites was closely related to the levels of AOS induced by different concentrations of ABA in leaves of maize seedlings (Fig. 1, 4, 6). These findings support the hypothesis mentioned above and suggest further that the generation of ABA-induced AOS may trigger the response of whole antioxidative defence systems against oxidative stress.
Although AOS may act as secondary messengers to regulate gene expression and protein biosynthesis involved in the stress defences, these may only occur under subtoxic condition. AOS at higher concentrations are still cytotoxic. Our data showed that 10 to 100 µM ABA treatment significantly induced the increase in the levels of O2– and H2O2 and enhanced the capacity of protective systems in leaves of maize seedlings. Such an enhancement in the antioxidant defences is sufficient to scavenge these increased AOS, and the oxidative damage expressed as lipid peroxidation, protein oxidation, and plasma membrane leakage did not take place. Treatment with 1,000 µM ABA led to a more abundant generation of O2– and H2O2. The capacity of antioxidative defence systems was still maintained at a higher level, but no longer further enhanced when compared with the treatment of 100 µM ABA. The contents of ASC and GSH had no changes in leaves treated with 1,000 µM ABA for 12 and 24 h, compared to the control. A marked oxidative damage occurred in leaves of maize seedlings. These suggest that the superoptimal concentration of 1,000 µM ABA may have induced a severe oxidative stress that cannot be controlled by the enhanced antioxidative defence systems.
Meanwhile, the toxicity caused by the excess generation of O2– and H2O2 may be further aggravated by the increase of catalytic Fe, which is critical for H2O2-dependent •OH production. This catalytic Fe may be released from Fe-protein such as phytoferritin (Iturbe-Ormaetxe et al. 1998). Catalytic Fe may interact in turn with O2– and H2O2 through a Haber-Weiss reaction to yield •OH, which is the strongest oxidizing agent known and is a major AOS responsible for modifications of macromolecules and cell damage (Bowler et al. 1992, Smirnoff 1993). The accumulation of catalytic Fe has been found in plant tissues exposed to water stress (Moran et al. 1994, Gogorcena et al. 1995, Iturbe-Ormaetxe et al. 1998) and senescence (Becana and Klucas 1992, Escuredo et al. 1996, Gogorcena et al. 1997, Evans et al. 1999). The accumulation of catalytic Fe may imply the generation of •OH. In contrast to O2– and H2O2, enzymes that scavenge •OH have not been identified (Shen et al. 1997). Its scavenging depends on the presence of antioxidative metabolites in vivo such as ASC, GSH and some compatible solutes (Smirnoff 1993, Shen et al. 1997). Our results showed that the contents of ASC and GSH, two important •OH scavengers, had no changes in leaves treated with 1,000 µM ABA, in contrast with a significant increase in the levels of ASC and GSH in leaves treated with 10 to 100 µM ABA. These suggest that the •OH radicals produced by catalytic Fe may be not controlled effectively and lead to an oxidative stress in leaves treated with 1,000 µM ABA. Therefore, O2– and H2O2 have dual effects in the response to oxidative stress in plants, as both toxins and triggers. Although we do not know the exact concentration range of these AOS as triggers, it is clear that a slight increase in the levels of AOS induced by ABA may be necessary for the stress tolerance and survival in plants exposed to environmental stresses.
There is only limited information about the mechanism with which ABA leads to the generation of AOS in plant cells. One speculation is that ABA-induced stomatal closing can cause the reduction in the availability of CO2 for photosynthesis, which may lead to the generation of AOS from the misdirecting of electrons in the photosystems (Bowler et al. 1992, Guan and Scandalios 1998a). However, in guard cells of Arabidopsis, ABA-induced H2O2 production and the H2O2-activated Ca2+ channels are important mechanisms for ABA-induced stomatal closing (Pei et al. 2000). Diphenyleneiodonium chloride (DPI), a well-known inhibitor of trans-plasma membrane NAD(P)H oxidase (O2– synthase) (Levine et al. 1994, Orozco-Cárdenas and Ryan 1999, Orozco-Cárdenas et al. 2001), blocked the production of AOS and partially inhibited ABA-induced stomatal closing. These results indicate that an oxidative burst occurred prior to the stomatal closing in the guard cells exposed to ABA treatment and suggest that a trans-plasma membrane NAD(P)H oxidase (O2– synthase) might be involved in the ABA signaling. We have preliminary data indicating that DPI can almost completely prevent the increased production of O2– and H2O2 induced by ABA, and fully inhibited the enhancement in the activities of antioxidant enzymes (Jiang and Zhang, unpublished observation). This matter is presently under further investigation.
In conclusion, treatment with lower concentrations (10 to 100 µM) of ABA led to a relatively slight increase in the levels of O2– and H2O2 and induced the capacity of whole antioxidant defence systems against oxidative stress. Although treatment with higher concentration of ABA (1,000 µM) maintained the capacity of antioxidant defence at a higher level, a marked oxidative damage occurred. The generation of an excessive O2– and H2O2 and the increase in the content of catalytic Fe, hence •OH production, along with the relatively low contents of ASC and GSH, provide a cogent explanation for the damage to lipids and proteins that occurs in leaves treated with 1,000 µM ABA.
Materials and Methods
Plant material
Seeds of maize (Zea mays L. cv. Yidan No. 22) were surface-sterilized with 0.2% HgCl2 for 10 min and germinated at 28°C for 1 d. The seedlings were grown in trays of sand in a greenhouse at a temperature of 25–30°C, photosynthetic active radiation (PAR) of 400 µmol m–2 s–1 (enhanced with high-pressure sodium lamps) and photoperiod of 14/10 h (day/night), and watered daily. When the second leaf was fully expanded, they were collected and used for investigations.
ABA treatments
Detached leaves were cut into leaf segments (3 cm in length), rinsed with distilled water and then floated in solutions containing 0, 10, 100, and 1,000 µM ABA, at 25°C with a light intensity of 80 µmol m–2 s–1. Leaf segments were vacuum-infiltrated with these solutions before the incubation. After different intervals samples were taken out, immediately frozen in liquid N2, and then stored at –80°C for further analysis.
Assays of antioxidant enzyme activities
Frozen leaf segments (0.5 g) were crushed into fine powder in a mortar and pestle under liquid N2. Soluble proteins were extracted by homogenizing the powder in 10 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone, with the addition of 1 mM ASC in the case of APX assay. The homogenate was centrifuged at 15,000×g for 20 min at 4°C and the supernatant was used for the following enzyme assays. Protein content was determined according to the method of Bradford (1976) with bovine serum albumin as standard.
Total SOD (EC1.15.1.1) activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) according to the method of Giannopolitis and Ries (1977). The 3 ml reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8), 13 mM methionine, 75 µM NBT, 2 µM riboflavin, 0.1 mM EDTA and 100 µl enzyme extract. The reaction mixtures were illuminated for 15 min at light intensity of 5,000 lux. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the reduction of NBT as monitored at 560 nm.
CAT (EC1.11.1.6) activity was determined by following the consumption of H2O2 (extinction coefficient 39.4 mM–1 cm–1) at 240 nm for 3 min (Aebi 1984). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 10 mM H2O2 and 200 µl of enzyme extract in a 3 ml volume.
GR (EC1.6.4.2) activity was determined by following the oxidation of NADPH at 340 nm (extinction coefficient 6.2 mM–1 cm–1) for 3 min in 1 ml of an assay mixture containing 50 mM potassium phosphate buffer (pH 7.8), 2 mM Na2EDTA, 0.15 mM NADPH, 0.5 mM GSSG and 200 µl of enzyme extract. The reaction was initiated by adding NADPH. Corrections were made for the background absorbance at 340 nm, without NADPH (Schaedle and Bassham 1977).
APX (EC1.11.1.11) activity was determined by following the decrease in A290 (extinction coefficient 2.8 mM–1 cm–1) for 1 min in 1 ml of a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ASC, 0.1 mM H2O2 and 200 µl of enzyme extract. The reaction was started by adding enzyme extract. Correction was done for the low, non-enzymatic oxidation of ASC by H2O2 (Nakano and Asada 1981).
Determination of non-enzymatic antioxidants
ASC was extracted from 0.5 g leaf segments with 10 ml of 5% trichloroacetic acid. ASC was determined in the supernatant after centrifugation at 14,000×g for 10 min, using a method based on the reduction of ferric ion to ferrous ion with ASC in acid solution and then the formation of the red chelate between ferrous ion and bathophenanthroline, which absorbs at 530 nm, as described by Arakawa et al. (1981). A standard curve covering the range of 0–25 nmol ASC was used to calculate the concentration of ASC in the samples.
Total glutathione (GSH + GSSG) was extracted from 1g leaf segments with 10 ml of 5% sulfosalicylic acid and centrifuged at 14,000×g for 10 min. Total glutathione and GSSG were determined by the 5,5′-dithiobis-(2-nitrobenzoic acid)-GR recycling procedure (Griffiths 1980). Changes in absorbance of the reaction mixtures were measured at 412 nm and the total glutathione content was calculated from a standard curve with GSH. GSSG was determined after removal of GSH by 2-vinylpyridine derivatization. A specific standard curve with GSSG was used. GSH was determined by subtraction of GSSG from the total glutathione content.
α-TOC was extracted as described by Munné-Bosch et al. (1999). Leaf segments (0.5 g) were ground in liquid N2 using a pestle and mortar with ice-cold 5 ml of methanol containing 1% ASC. The macerate was homogenized and α-TOC was extracted by vigorous mixing for 1 min with 4 ml of hexane. After the samples were centrifuged at 1,500×g for 10 min, the upper hexane layer was carefully removed and evaporated to dryness under N2. Samples were dissolved in 2 ml of methanol and injected into a HPLC apparatus in 100 µl portions. α-TOC was separated at room temperature using an Ultrasphere ODS 5 µm column (250×4.6 mm) with 100% methanol as an eluant at a flow rate of 1.5 ml min–1. UV detection was carried out at 295 nm and fluorescence detection was carried out at an excitation wavelength of 298 nm and an emission at 328 nm. Pure (±)-α-TOC was used as a standard.
Carotenoid (CAR) and chlorophyll were spectrophotometrically measured in 80% acetone extract, as described by Lichtenthaler (1987).
Determination of superoxide radical, hydrogen peroxide and catalytic Fe
O2– was measured as described by Elstner and Heupel (1976) by monitoring the nitrite formation from hydroxylamine in the presence of O2–, with some modifications. One g of frozen leaf segments was homogenized with 3 ml of 65 mM potassium phosphate buffer (pH 7.8) and centrifuged at 5,000×g for 10 min. The incubation mixture contained 0.9 ml of 65 mM phosphate buffer (pH 7.8), 0.1 ml of 10 mM hydroxylamine hydrochloride, and 1 ml of the supernatant. After incubation at 25°C for 20 min, 17 mM sulfanilamide and 7 mM α-naphthylamine were added to the incubation mixture. After reaction at 25°C for 20 min, ethyl ether in the same volume was added and centrifuged at 1,500×g for 5 min. The absorbance in the aqueous solution was read at 530 nm. A standard curve with NO2– was used to calculate the production rate of O2– from the chemical reaction of O2– and hydroxylamine.
The content of H2O2 was measured by monitoring the A415 of the titanium-peroxide complex following the method described by Brennan and Frenkel (1977). Absorbance values were calibrated to a standard curve generated with known concentrations of H2O2.
For assay of catalytic Fe, leaf segments were extracted with Chelex-treated 50 mM potassium phosphate buffer (pH 7.0) and the homogenate was centrifuged at 15,000×g for 20 min and further fractionated using Centricon-3 membranes (Amicon). The concentration of catalytic Fe in the <3-kDa fraction was measured as bleomycin-dependent DNA damage (Evans and Halliwell 1994) using 50 µl of sample, incubation at 37°C for 1 h, and free Fe3+ as standard.
Determination of indices of oxidative damage
Oxidative damage to lipids was estimated by measuring the content of MDA in leaf segment homogenates, prepared in 10% trichloroacetic acid containing 0.65% 2-thiobarbituric acid (TBA) and heated at 95°C for 25 min, as described by Hodges et al. (1999). MDA content was calculated by correcting for compounds other than MDA which absorb at 532 nm by subtracting the absorbance at 532 nm of a solution containing plant extract incubated without TBA from an identical solution containing TBA.
Oxidative damage to proteins was estimated as the content of carbonyl groups (Reznick and Packer 1994). Leaf segments (0.5 g) were homogenized with 3 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 2.5 µg ml–1 each of the protease inhibitors phenylmethylsulfonyl fluoride, leupeptin, pepstatin, and aprotinin. The homogenate was centrifuged at 15,000×g for 25 min and the supernatant was freed from contaminating nucleic acids by treatment with streptomycin sulfate, and the protein carbonyl content was determined by reaction with 2,4-dinitrophenylhydrazine.
The relative intactness of plasma membrane was measured as the leakage percentage of electrolytes, as described by Gong et al. (1998). Fresh leaves (0.3 g) were cut into pieces of 1 cm length and placed in test tubes containing 30 ml distilled deionized water. The tubes were incubated in a water bath at 30°C for 2 h and the initial electrical conductivity of the medium (EC1) was measured. The samples were boiled at 100°C for 15min to release all electrolytes, cooled and the final electrical conductivity (EC2) was measured. The leakage percentage of electrolytes was calculated by using the formula: EC1/EC2×100.
Acknowledgements
We are grateful for grants obtained from FRG of HKBU, RGC of HKUGC and AOE of Chinese University of HK. M J is grateful for the postgraduate studentship from HKBU.
Corresponding author: E-mail, jzhang@hkbu.edu.hk; Fax, +852-2339-5995.
Fig. 1 Effects of ABA at different concentrations on levels of O2– (A) and H2O2 (B) in leaves of maize seedlings. Values are the means of three different experiments. Error bars represent SE with n = 6. Asterisks indicate the significance of difference at P<0.05 level by Student’s t-test when compared to control.
Fig. 2 Time course of ABA action on levels of O2– (A) and H2O2 (B) in leaves of maize seedlings. Values are the means of three different experiments. Error bars represent SE with n = 6. Asterisks indicate the significance of difference at P<0.05 level by Student’s t-test when compared to control.
Fig. 3 Effects of ABA at different concentrations on the content of catalytic Fe in leaves of maize seedlings. Values are the means of three different experiments. Error bars represent SE with n = 6. Asterisks indicate the significance of difference at P<0.05 level by Student’s t-test when compared to control.
Fig. 4 Effects of ABA at different concentrations on activities of SOD (A), CAT (B), APX (C) and GR (D) in leaves of maize seedlings. Values are the means of three different experiments. Error bars represent SE with n = 6. Asterisks indicate the significance of difference at P<0.05 level by Student’s t-test when compared to control.
Fig. 5 Time course of ABA action on activities of SOD (A), CAT (B), APX (C) and GR (D) in leaves of maize seedlings. Values are the means of three different experiments. Error bars represent SE with n = 6. Asterisks indicate the significance of difference at P<0.05 level by Student’s t-test when compared to control.
Fig. 6 Effects of ABA at different concentrations on contents of ASC (A), GSH (B), α-TOC (C) and CAR (D) in leaves of maize seedlings. Values are the means of three different experiments. Error bars represent SE with n = 6. Asterisks indicate the significance of difference at P<0.05 level by Student’s t-test when compared to control.
Fig. 7 Effects of ABA at different concentrations on lipid peroxidation (expressed as MDA) (A), protein oxidation (total carbonyl groups) (B) and plasma membrane leakage (electrolyte leakage %) (C) in leaves of maize seedlings. Values are the means of three different experiments. Error bars represent SE with n = 6. Asterisks indicate the significance of difference at P<0.05 level by Student’s t-test when compared to control.
Abbreviations
- AOS
active oxygen species
- APX
ascorbate peroxidase
- ASC
ascorbate
- CAR
carotenoid
- CAT
catalase
- GP
glutathione peroxidase
- GPX
guaiacol peroxidase
- GR
glutathione reductase
- GST
glutathione-S-transferase
- MDA
malondialdehyde
- SOD
superoxide dismutase
- α-TOC
α-tocopherol.
References
Anderson, M.D., Prasad, T.K., Martin, B.A. and Stewart, C.R. (
Arakawa, N., Tsutsumi, K., Sanceda, N.G., Kurata, T. and Inagaki, C. (
Becana, M. and Klucas, R.V. (
Bellaire, B.A., Carmody, J., Braud, J., Gossett, D.R., Banks, S.W., Lucas, M.C. and Fowler, T.E. (
Bowler, C., Van Montagu, M. and Inzé, D. (
Bradford, M.M. (
Brennan, T. and Frenkel, C. (
Bueno, P., Piqueras, A., Kurepa, J., Savouré, A., Verbruggen, N., Van Montagu, M. and Inzé, D. (
Chandler, P.M. and Robertson, M. (
Dat, J.F., Lopez-Delgado, H., Foyer, C.H. and Scott, I.M. (
Dat, J.F., Lopez-Delgado, H., Foyer, C.H. and Scott, I.M. (
Elstner, E.F. and Heupel, A. (
Escuredo, P.R., Minchin, F.R., Gogorcena, Y., Iturbe-Ormaetxe, I., Klucas, R.V. and Becana, M. (
Evans, P.J., Gallesi, D., Mathieu, C., Jesus Hernandez, M., de Felipe, M., Halliwell, B. and Puppo, A. (
Evans, P.J. and Halliwell, B. (
Giannopolitis, C.N. and Ries, S.K. (
Gogorcena, Y., Gordon, A.J., Escuredo, P.R., Minchin, F.R., Witty, J.F., Moran, J.F. and Becana, M. (
Gogorcena, Y., Iturbe-Ormaetxe, I., Escuredo, P.R. and Becana, M. (
Gong, M., Li, Y.J. and Chen, S.Z. (
Griffiths, O.W. (
Guan, L. and Scandalios, J.G. (
Guan, L. and Scandalios, J.G. (
Guan, L., Zhao, J. and Scandalios, J.G. (
Hare, P.D., Cress, W.A. and Van Staden, J. (
Hodges, D.M., DeLong, J.M., Forney, C.F. and Prange, R.K. (
Iturbe-Ormaetxe, I., Escuredo, P.R., Arrese-Igor, C. and Becana, M. (
Kaminaka, H., Morita, S., Tokumoto, M., Masumura T. and Tanaka, K. (
Kawano, T., Sahashi, N., Takahashi, K., Uozumi, N. and Muto, S. (
Levine, A., Tenhaken, R., Dixon, R. and Lamb, C. (
Lichtenthaler, H.K. (
Moran, J.F., Becana, M., Iturbe-Ormaetxe, I., Frechillo, S., Klucas, R.V. and Aparicio-Tejo, P. (
Morita, S., Kaminaka, H., Masumura, T. and Tanaka, K. (
Munné-Bosch, S., Schwarz, K. and Alegre, L. (
Nakano, Y. and Asada, K. (
Nambara, E., Kawaide, H., Kamiya, K. and Naito, S. (
Orozco-Cárdenas, M.L., Narváez-Vásquez, J. and Ryan, C.A. (
Orozco-Cárdenas, M.L. and Ryan, C.A. (
Pei, Z.M., Murata, N., Benning, G., Thomine, S., Klüsener, B., Allen, G.J., Grill, E. and Schroeder, J.I. (
Prasad, T.K., Anderson, M.D., Martin, B.A. and Stewart, C.R. (
Prasad, T.K., Anderson, M.D. and Stewart, C.R. (
Rao, M.V., Paliyath, G., Ormrod, D.P., Murr, D.P. and Watkins, C.B. (
Reznick, A.Z. and Packer, L. (
Sakamoto, A., Okumura, T., Kaminata, H., Sumi, K. and Tanaka, K. (
Schaedle, M. and Bassham, J.A. (
Shen, B., Jensen, R.G. and Bohnert, H.J. (
Shinozaki, K. and Yamaguchi-Shinozaki, K. (
Siddiqui, N.U., Chung, H.-J., Thomas, T.L. and Drew, M.C. (
Smirnoff, N. (
Taylor, I.B., Burbidge, A. and Thompson, A.J. (
Williamson, J.D. and Scandalios, J.G. (