Suppressive Interaction Approach for Masking Stale Note of Instant Ripened Pu-Erh Tea Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sensory Evaluation of the Aroma Profile
2.2. GC-MS Analysis of Volatile Constituents
2.3. GC-O Analysis of Aroma-Active Volatiles
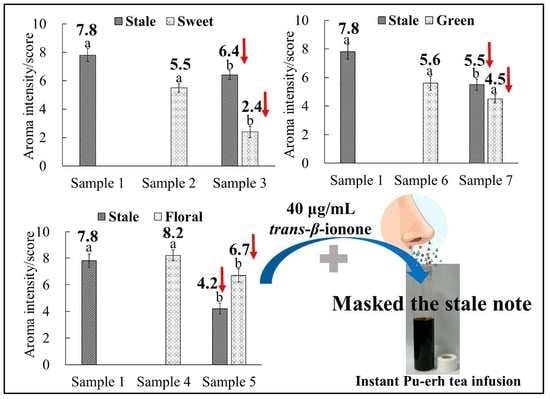
2.4. Investigation of the Suppressive Interaction between the Stale Note and Other Notes
2.5. Validation of Masking the Stale Note in Instant Ripened Pu-Erh Tea Infusion
3. Materials and Methods
3.1. Instant Ripened Pu-Erh Tea
3.2. Chemical Standards and Reagents
3.3. Extraction of Volatiles from Instant Ripened Pu-Erh Tea
3.4. Sensory Evaluation of the Aroma Profile
3.5. GC-MS Analysis of the Volatile Constituents
3.6. GC-O Analysis of the Aroma-Active Volatiles
3.7. Investigation of the Suppressive Interaction between the Stale Note and Other Notes
3.8. Validation of Masking the Stale Note in Instant Ripened Pu-Erh Tea Infusion
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shekoohiyan, S.; Ghoochani, M.; Mohagheghian, A.; Mahvi, A.H.; Yunesian, M.; Nazmara, S. Determination of lead, cadmium and arsenic in infusion tea cultivated in north of Iran. Iran. J. Environ. Health Sci. Eng. 2012, 9, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraujalyte, V.; Pelvan, E.; Alasalvar, C. Volatile compounds and sensory characteristics of various instant teas produced from black tea. Food Chem. 2016, 194, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.F.; Chen, S.H.; Chen, H.; Wang, Y.; Wang, Y.; Hochstetter, D.; Xu, P. Studies on the bioactivity of aqueous extract of pu-erh tea and its fractions: In vitro antioxidant activity and α-glycosidase inhibitory property, and their effect on postprandial hyperglycemia in diabetic mice. Food Chem. Toxicol. 2013, 53, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K. Cancer chemoprevention by tea polyphenols through modulating signal transduction pathways. Arch. Pharmacal Res. 2002, 25, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Lucysun, H. Polyphenol contents of Pu-erh teas and their abilities to inhibit cholesterol biosynthesis in Hep G2 cell line. Food Chem. 2008, 111, 67–71. [Google Scholar] [CrossRef]
- Wu, S.C.; Yen, G.C.; Wang, B.S.; Chiu, C.K.; Yen, W.J. Antimutagenic and antimicrobial activities of Pu-erh tea. LWT-Food Sci.Technol. 2007, 40, 506–512. [Google Scholar] [CrossRef]
- Someswararao, C.; Srivastav, P.P. A novel technology for production of instant tea powder from the existing black tea manufacturing process. Innov. Food Sci. Emerg. Technol. 2012, 16, 143–147. [Google Scholar] [CrossRef]
- Du, L.P.; Li, J.X.; Li, W.; Li, Y.F.; Li, T.; Xiao, D.G. Characterization of volatile compounds of Pu-erh tea using solid-phase microextraction and simultaneous distillation-extraction coupled with gas chromatography-mass spectrometry. Food Res. Int. 2014, 57, 61–70. [Google Scholar] [CrossRef]
- Du, L.P.; Wang, C.; Li, J.X.; Xiao, D.G.; Li, C.W.; Xu, Y.Q. Optimization of headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry for detecting methoxyphenolic compounds in Pu-erh tea. J. Agric. Food Chem. 2013, 61, 561–568. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Wang, C.; Li, C.W.; Liu, S.H.; Zhang, C.X.; Li, L.W.; Jiang, D.H. Characterization of aroma-active compounds of Pu-erh tea by headspace solid-phase microextraction (HS-SPME) and simultaneous distillation-extraction (SDE) coupled with GC-Olfactometry and GC-MS. Food Anal. Methods 2016, 9, 1188–1198. [Google Scholar] [CrossRef]
- Lv, H.P.; Zhong, Q.S.; Lin, Z.; Wang, L.; Tan, J.F.; Guo, L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC–olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: A flavour chemistry view. Part 1: Intensity and detectability. A review. Flavour Fragr. J. 2012, 27, 124–140. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Luo, J.; Niu, Y.W.; Wang, P.P.; Wang, R.L.; Sun, X.X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Int. 2019, 116, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lytra, G.; Tempere, S.; Le Floch, A.; De Revel, G.; Barbe, J. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef] [PubMed]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.C.; Chen, F.; Wang, L.Y.; Niu, Y.W.; Xiao, Z.B. Evaluation of the synergism among volatile compounds in Oolong tea infusion by odour threshold with sensory analysis and E-nose. Food Chem. 2017, 221, 1484–1490. [Google Scholar] [CrossRef]
- Ito, Y.; Kubota, K. Sensory evaluation of the synergism among odorants present in concentrations below their odor threshold in a Chinese jasmine green tea infusion. Mol. Nutr. Food Res. 2005, 49, 61–68. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Perezcacho, P.R.; Jabalpurwala, F.; Armstrong, R.N. Historical review of citrus flavor research during the past 100 years. J. Agric. Food Chem. 2009, 57, 8115–8124. [Google Scholar] [CrossRef]
- Lv, S.D.; Wu, Y.S.; Zhou, J.S.; Li, C.M.; Li, C.; Xu, Y.Q.; Liu, S.H.; Wang, C.; Meng, Q.X. The study of fingerprint characteristics of Dayi Pu-erh tea using a fully automatic HS-SPME/GC–MS and combined chemometrics method. PLoS ONE 2014, 9, e116428. [Google Scholar] [CrossRef]
- Gulati, A.; Ravindranath, S.D. Seasonal variations in quality of Kangra tea (Camellia sinensis (L) O Kuntze) in Himachal Pradesh. J. Sci. Food Agric. 1996, 71, 231–236. [Google Scholar] [CrossRef]
- Daan, S.; Davidp, D.S.; Bregt, U.; Filip, D.; Freddyr, D. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar]
- Atanasova, B.; Thomas-Danguin, T.; Langlois, D.; Nicklaus, S.; Etievant, P. Perceptual interactions between fruity and woody notes of wine. Flavour Fragr. J. 2004, 19, 476–482. [Google Scholar] [CrossRef]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Peiyou, Q.; Tingjun, M.; Li, W.; Fang, S.; Guixing, R. Identification of tartary buckwheat tea aroma compounds with gas chromatography-mass spectrometry. J. Food Sci. 2011, 76, S401–S407. [Google Scholar]
- Ma, Q.L.; Hamid, N.; Oey, I.; Kantono, K.; Faridnia, F.; Yoo, M.; Farouk, M. Effect of chilled and freezing pre-treatments prior to pulsed electric field processing on volatile profile and sensory attributesof cooked lamb meats. Innov. Food Sci. Emerg. Technol. 2016, 37, 359–374. [Google Scholar] [CrossRef]
- Baylin, F.; Moulton, D.G. Adaptation and cross-adaptation to odor stimulation of olfactory receptors in the tiger salamander. J. Gen. Physiol. 1979, 74, 37–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, G.; Leray, I.; Grebert, D.; Antoine, S.; Acquistapace, A.; Muscat, A.; Boukadiri, A.; Mir, L.M. Structurally related odorant ligands of the olfactory receptor OR51E2 differentially promote metastasis emergence and tumor growth. Oncotarget 2017, 8, 4330–4341. [Google Scholar] [CrossRef] [Green Version]
- Du, X.F.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cultivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef]
- Cheong, M.W.; Chong, Z.S.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Characterisation of calamansi (Citrus microcarpa). Part I: Volatiles, aromatic profiles and phenolic acids in the peel. Food Chem. 2012, 134, 686–695. [Google Scholar] [CrossRef]
- Ni, H.; Hong, P.; Ji, H.F.; Sun, H.; Chen, Y.H.; Xiao, A.F.; Chen, F. Comparative analyses of aromas of fresh, naringinase-treated and resin-absorbed juices of pummelo by GC-MS and sensory evaluation. Flavour Fragr. J. 2015, 30, 245–253. [Google Scholar] [CrossRef]
- Liu, C.H.; Jiang, D.; Cheng, Y.J.; Deng, X.; Chen, F.; Fang, L.; Ma, Z.; Xu, J. Chemotaxonomic study of citrus, poncirus and fortunella genotypes based on peel oil volatile compounds—Deciphering the genetic origin of Mangshanyegan (Citrus nobilis Lauriro). PLoS ONE 2013, 8, e58411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |




| No. | Volatiles | Rtx-5MS | Characteristic Ion Fragment | Std c | Calibration Equation d | Range (μg/mL) | R2 | CF e | Concentration (μg/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|
| a RI1 | b RI2 | |||||||||
| Aldehydes | ||||||||||
| 1 | Benzeneacetaldehyde | 1045 | 1045 | 91 92 120 | MS, Std | Y = 1.39737X − 0.05430 | 0.025–5 | 0.9995 | 0.719 | 0.83 ± 0.04 |
| 2 | Safranal | 1203 | 1203 | 107 91 121 | MS, Std | Y = 2.02835X − 0.25929 | 0.025–5 | 0.9991 | 0.504 | 0.13 ± 0.01 |
| 3 | 1-ethyl-1H-Pyrrole-2-carboxaldehyde | 1051 | 1052 | 39 94 123 | MS | c | - | - | - | 0.70 ± 0.01 |
| Alcohols | ||||||||||
| 4 | Benzyl alcohol | 1037 | 1037 | 108 79 107 | MS, Std | Y = 0.19632X − 0.43109 | 0.025–5 | 0.9970 | 0.541 | 2.16 ± 0.0.11 |
| 5 | Linalool oxide I | 1074 | 1074 | 59 94 43 | MS, Std | Y = 4.30922X − 0.07267 | 0.025–5 | 0.9997 | 0.233 | 0.67 ± 0.01 |
| 6 | Linalool oxide II | 1090 | 1088 | 59 43 94 | MS, Std | Y = 2.84204X − 0.06826 | 0.025–5 | 0.9997 | 0.354 | 0.79 ± 0.01 |
| 7 | Linalool | 1102 | 1101 | 71 41 93 | MS, Std | Y = 2.29175X − 0.17466 | 0.025–5 | 0.9996 | 0.719 | 1.44 ± 0.03 |
| 8 | Hotrienol | 1107 | 1104 | 71 82 152 | MS | c | - | - | - | 0.27 ± 0.01 |
| 9 | 3-Octen-2-ol | 1110 | 1114 | 43 71 | MS, Std | Y = 3.63218X − 0.20835 | 0.025–5 | 0.9991 | 0.277 | 0.73 ± 0.01 |
| 10 | Phenylethyl alcohol | 1116 | 1116 | 91 92 122 | MS, Std | Y = 7.61778X − 0.53450 | 0.025–5 | 0.9995 | 0.132 | 0.21 ± 0.01 |
| 11 | Linalool oxide III * | 1172 | 1173 | 43 94 67 | MS | Y = 2.84204X − 0.06826 | 0.025–5 | 0.9997 | 0.354 | 1.55 ± 0.03 |
| 12 | Menthol | 1176 | 1178 | 71 81 95 | MS, Std | Y = 0.24437X − 0.01346 | 0.025–5 | 0.9996 | 4.122 | 2.00 ± 0.17 |
| 13 | Linalool oxide IV * | 1178 | 1175 | 43 94 67 | MS | Y = 2.84204X − 0.06826 | 0.025–5 | 0.9997 | 0.354 | 4.58 ± 0.04 |
| 14 | α-Terpineol | 1193 | 1195 | 59 93 121 | MS, Std | Y = 1.24692X − 0.07854 | 0.025–5 | 0.9994 | 0.808 | 1.17 ± 0.01 |
| 15 | Nerol | 1232 | 1232 | 69 41 93 | MS, Std | Y = 2.02835X − 0.25929 | 0.025–5 | 0.9992 | 0.413 | 0.24 ± 0.01 |
| 16 | Syringol | 1249 | 1239 | 93 139 154 | MS | c | - | - | - | 0.59 ± 0.02 |
| 17 | Geraniol | 1259 | 1259 | 69 41 68 | MS, Std | Y = 1.66627X − 0.18129 | 0.025–5 | 0.9994 | 0.603 | 0.78 ± 0.02 |
| 18 | 2,4-Ditert-butylphenol | 1518 | 1513 | 191 57 206 | MS, Std | Y = 17.5470X + 0.15020 | 0.005–5 | 0.9998 | 0.057 | 0.01 ± 0.00 |
| Ketones | ||||||||||
| 19 | 2-Hexanone | - | 792 | 43 58 57 | MS, Std | Y = 0.24039X − 0.26212 | 0.025–5 | 0.9990 | 0.264 | 0.94 ± 0.04 |
| 20 | 4-Oxoisophorone | 1146 | 1147 | 68 96 102 | MS, Std | Y = 0.56877X − 0.02251 | 0.025–5 | 0.9996 | 1.759 | 0.31 ± 0.01 |
| 21 | trans-β-Ionone | 1491 | 1490 | 177 43 41 | MS, Std | Y = 7.78433X − 0.12726 | 0.025–5 | 0.9995 | 0.129 | 0.16 ± 0.01 |
| Esters | ||||||||||
| 22 | Methyl salicylate | 1197 | 1197 | 39 92 120 | MS, Std | Y = 1.07835X − 0.05296 | 0.025–5 | 0.9990 | 2.416 | 0.05 ± 0.01 |
| 23 | Dihydroactinidiolide | 1538 | 1538 | 111 43 137 | MS, Std | Y = 0.04390X − 0.07338 | 20–500 | 0.9969 | 2.210 | 188.04 ± 3.35 |
| Methoxybenzenes | ||||||||||
| 24 | 1,2-Dimethoxybenzene | 1151 | 1149 | 138 95 123 | MS, Std | Y = 0.20751X − 0.27376 | 5–35 | 0.9976 | 0.430 | 15.54 ± 0.23 |
| 25 | 3,4-Dimethoxytoluene | 1243 | 1246 | 152 137 109 | MS, Std | Y = 0.16900X − 0.15431 | 0.05–10 | 0.9969 | 0.533 | 6.49 ± 0.06 |
| 26 | 1,2,3-Trimethoxybenzene | 1321 | 1315 | 117 90 89 | MS, Std | Y = 0.12953X − 0.00718 | 20–500 | 0.9997 | 7.774 | 260.53 ± 3.92 |
| 27 | 1,2,4-Trimethoxybenzene | 1378 | 1378 | 168 103 110 | MS, Std | Y = 2.22856X − 0.01142 | 5–35 | 1.0000 | 0.441 | 4.86 ± 0.12 |
| 28 | 1,2,3-Trimethoxy-5-methyl-benzene * | 1410 | 1410 | 168 103 125 | MS | Y = 2.22856X − 0.01142 | 5–35 | 1.0000 | 0.441 | 5.32 ± 0.09 |
| 29 | 1,2,3,4-Tetramethoxybenzene | 1453 | 1449 | 97 140 198 | MS | c | - | - | - | 2.10 ± 0.06 |
| Others | MS | |||||||||
| 30 | 1-Ethylpyrrole | 815 | 815 | 80 95 67 | MS, Std | Y = 1.01083X − 0.00793 | 0.025–5 | 0.9996 | 0.990 | 0.40 ± 0.01 |
| 31 | Indole | 1299 | 1300 | 117 90 89 | MS, Std | Y = 4.59138X − 0.47327 | 0.025–5 | 0.9994 | 0.221 | 0.26 ± 0.01 |
| 32 | <n->Hexadecanoic acid | - | 1962 | 73 256 | MS | c | - | - | - | 7.20 ± 0.08 |
| No. | a RI3 | b RI4 | Volatiles | Odor Description | FD |
|---|---|---|---|---|---|
| Aldehydes | |||||
| 1 | 1655 | 1650 | Benzeneacetaldehyde | Green | 16 |
| 2 | 1204 | 1203 | Safranal | Green | 1 |
| 3 | 1619 | 1616 | 1-ethyl-1H-pyrrole-2-carboxaldehyde | Green | 4 |
| Alcohols | |||||
| 4 | 1878 | 1877 | Benzyl alcohol | Sweet *, roasted | 4 |
| 5 | 1439 | 1435 | Linalool oxide I | Sweet | 4 |
| 6 | 1468 | 1470 | Linalool oxide II | Sweet | 16 |
| 7 | 1543 | 1549 | Linalool | Floral | 16 |
| 8 | 1915 | 1919 | Phenylethyl alcohol | Floral | 4 |
| 9 | 1753 | 1750 | Linalool oxide III | Sweet | 16 |
| 10 | 1636 | 1632 | Menthol | Green | 1 |
| 11 | 1780 | 1775 | Linalool oxide IV | Sweet | 16 |
| 12 | 1718 | 1715 | α-Terpineol | Wood | 4 |
| 13 | - | 2321 | 2,4-Ditert-butylphenol | Green | 1 |
| Ketones | |||||
| 14 | 1105 | 1102 | 2-Hexanone | Fruity, floral | 1 |
| 15 | 1955 | 1954 | trans-β-Ionone | Floral | 16 |
| Esters | |||||
| 16 | 1763 | 1759 | Methyl salicylate | Sweet | 1 |
| Methoxybenzenes | |||||
| 17 | 1731 | 1731 | 1,2-Dimethoxybenzene | Stale | 4 |
| 18 | 1807 | 1806 | 3,4-Dimethoxytoluene | Stale | 1 |
| 19 | 1961 | 1955 | 1,2,3-Trimethoxybenzene | Stale | 16 |
| 20 | - | 2094 | 1,2,4-Trimethoxybenzene | Stale | 16 |
| 21 | - | 2041 | 1,2,3-Trimethoxy-5-methyl-benzene | Stale | 16 |
| 22 | - | 2321 | 1,2,3,4-Tetramethoxybenzene | Green | 4 |
| Others | |||||
| 23 | 1179 | 1178 | 1-Ethylpyrrole | Roasted | 4 |
| 24 | - | 2435 | Indole | Floral | 4 |
| Aroma Characteristic | Standard Solution | Concentration for Aroma Intensity of 0 Scores (μL/L) | Concentration for Aroma Intensity of 9 Scores (μL/L) |
|---|---|---|---|
| Green | Benzeneacetaldehyde | 100 | 900 |
| Floral | Linalool | 20 | 180 |
| Sweet | Linalool oxide | 10 | 90 |
| Roasted | 2-Ethyl-3-methylpyrazine | 130 | 1170 |
| Stale | 1,2,4-Trimethoxybenzene | 1147 | 10323 |
| Samples | Aroma Description | Compound Composition and Concentration |
|---|---|---|
| Sample 1# | Stale | Mixture of 15.54 μg/mL of 1,2-dimethoxybenzene, 260.53 μg/mL of 1,2,3-trimethoxybenzene and 4.86 μg/mL of 1,2,4-trimethoxybenzene |
| Sample 2# | Sweet | Mixture of 0.67 μg/mL of linalool oxide I and 0.79 μg/mL of linalool oxide II |
| Sample 3# | Stale + sweet | Mixture of samples 1# and samples 2# |
| Sample 4# | Floral | Mixture of 1.44 μg/mL of linalool, 0.16 μg/mL of trans-β-ionone, 0.21 μg/mL of phenylethyl alcohol and 0.26 μg/mL of indole |
| Sample 5# | Stale + floral | Mixture of samples 1# and samples 4# |
| Sample 6# | Green | Mixture of 0.83 μg/mL of benzeneacetaldehyde |
| Sample 7# | Stale + green | Mixture of samples 1# and samples 6# |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Ni, H.; Qiu, X.-J.; Li, T.; Zhang, L.-Z.; Li, L.-J.; Jiang, Z.-D.; Li, Q.-B.; Chen, F.; Zheng, F.-P. Suppressive Interaction Approach for Masking Stale Note of Instant Ripened Pu-Erh Tea Products. Molecules 2019, 24, 4473. https://doi.org/10.3390/molecules24244473
Zhang T, Ni H, Qiu X-J, Li T, Zhang L-Z, Li L-J, Jiang Z-D, Li Q-B, Chen F, Zheng F-P. Suppressive Interaction Approach for Masking Stale Note of Instant Ripened Pu-Erh Tea Products. Molecules. 2019; 24(24):4473. https://doi.org/10.3390/molecules24244473
Chicago/Turabian StyleZhang, Ting, Hui Ni, Xu-Jian Qiu, Ting Li, Liang-Zhen Zhang, Li-Jun Li, Ze-Dong Jiang, Qing-Biao Li, Feng Chen, and Fu-Ping Zheng. 2019. "Suppressive Interaction Approach for Masking Stale Note of Instant Ripened Pu-Erh Tea Products" Molecules 24, no. 24: 4473. https://doi.org/10.3390/molecules24244473